MurralonginCAS# 53011-72-6 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 53011-72-6 | SDF | Download SDF |
| PubChem ID | 179620 | Appearance | Powder |
| Formula | C15H14O4 | M.Wt | 258.3 |
| Type of Compound | Coumarins | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
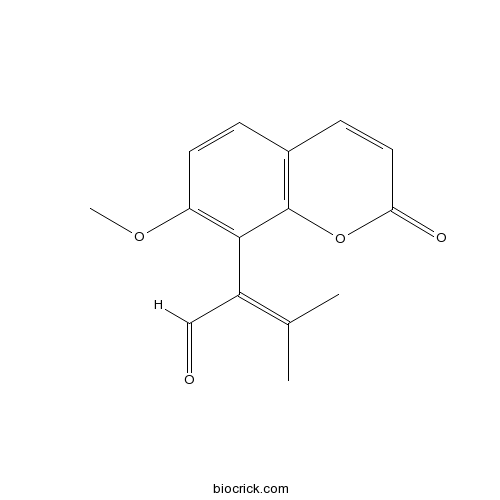
| Chemical Name | 2-(7-methoxy-2-oxochromen-8-yl)-3-methylbut-2-enal | ||
| SMILES | CC(=C(C=O)C1=C(C=CC2=C1OC(=O)C=C2)OC)C | ||
| Standard InChIKey | PBAZKMWQUBDDLZ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C15H14O4/c1-9(2)11(8-16)14-12(18-3)6-4-10-5-7-13(17)19-15(10)14/h4-8H,1-3H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Murralongin exhibits cytotoxicity against cholangiocarcinoma cell line, KKU-100. |

Murralongin Dilution Calculator

Murralongin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.8715 mL | 19.3573 mL | 38.7147 mL | 77.4293 mL | 96.7867 mL |
| 5 mM | 0.7743 mL | 3.8715 mL | 7.7429 mL | 15.4859 mL | 19.3573 mL |
| 10 mM | 0.3871 mL | 1.9357 mL | 3.8715 mL | 7.7429 mL | 9.6787 mL |
| 50 mM | 0.0774 mL | 0.3871 mL | 0.7743 mL | 1.5486 mL | 1.9357 mL |
| 100 mM | 0.0387 mL | 0.1936 mL | 0.3871 mL | 0.7743 mL | 0.9679 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Salinomycin
Catalog No.:BCC1916
CAS No.:53003-10-4
- CDI (1,1′-Carbonyldiimidazole)
Catalog No.:BCC2809
CAS No.:530-62-1
- Sinapic acid
Catalog No.:BCN3539
CAS No.:530-59-6
- Syringic acid
Catalog No.:BCN5699
CAS No.:530-57-4
- 2,6-Dimethoxy-1,4-benzoquinone
Catalog No.:BCN5698
CAS No.:530-55-2
- Deoxyvasicinone
Catalog No.:BCN5697
CAS No.:530-53-0
- L-Picein
Catalog No.:BCC8336
CAS No.:530-14-3
- Indomethacin
Catalog No.:BCC3794
CAS No.:53-86-1
- Dehydroepiandrosterone
Catalog No.:BCN2202
CAS No.:53-43-0
- Oxandrolone
Catalog No.:BCC5242
CAS No.:53-39-4
- Methylprednisolone acetate
Catalog No.:BCC9043
CAS No.:53-36-1
- Cocaine hydrochloride
Catalog No.:BCC5943
CAS No.:53-21-4
- T-5224
Catalog No.:BCC5383
CAS No.:530141-72-1
- Scutebarbatine J
Catalog No.:BCN8134
CAS No.:960302-85-6
- Morellic acid
Catalog No.:BCN3073
CAS No.:5304-71-2
- 9,13-Epidioxy-8(14)-abieten-18-oic acid
Catalog No.:BCN1426
CAS No.:5309-35-3
- Dichotomin
Catalog No.:BCN2836
CAS No.:53093-47-3
- Androsin
Catalog No.:BCN3842
CAS No.:531-28-2
- Coniferin
Catalog No.:BCN5700
CAS No.:531-29-3
- Scopolin
Catalog No.:BCN5701
CAS No.:531-44-2
- 7-Methoxycoumarin
Catalog No.:BCN2707
CAS No.:531-59-9
- Esculin
Catalog No.:BCN5904
CAS No.:531-75-9
- Coumarin-3-Carboxylic Acid
Catalog No.:BCC9220
CAS No.:531-81-7
- 4',7-Isoflavandiol
Catalog No.:BCN2855
CAS No.:531-95-3
C-7 oxygenated coumarins from the fruits of Micromelum minutum.[Pubmed:21544717]
Arch Pharm Res. 2011 Apr;34(4):527-31.
A new 7-oxygenated coumarin, 7-demethylMurralonginol isovalerate (1), and a new natural product, Murralonginol (2), together with seven known 7-oxygenated coumarins, Murralonginol isovalerate (3), Murralongin (4), micromelin (5), scopoletin (6), microminutin (7), murrangatin (8), and minumicrolin (9), were isolated from the fruits of Micromelum minutum. The structures of these compounds were established on the basis of their 1D and 2D NMR spectroscopic data. Among these isolates, compounds 2 and 4 - 9 exhibited cytotoxicity against cholangiocarcinoma cell line, KKU-100.
Coumarins from Galipea panamensis and Their Activity against Leishmania panamensis.[Pubmed:20423106]
J Nat Prod. 2010 May 28;73(5):1012-4.
Two new coumarin compounds (1 and 2), phebalosin (3), its derived artifact Murralongin (4), and murrangatin acetonide (5) were isolated from the leaves of Galipea panamensis. The structures of 1 and 2 were assigned as 7-{[(2R*)-3,3-dimethyloxiran-2-yl]methoxy}-8-[(2R*,3R*)-3-isopropenyloxiran-2-yl] -2H-chromen-2-one and 7-methoxy-8-(4-methyl-3-furyl)-2H-chromen-2-one, respectively, on the basis of their spectroscopic data (primarily NMR and MS). Compounds 1-3 were tested against axenic amastigote forms of Leishmania panamensis and displayed 50% effective concentrations (EC(50)) of 9.9, 10.5, and 14.1 microg/mL, respectively. These three compounds also displayed cytotoxicity (IC(50)) at concentrations of 9.7, 33.0, and 20.7 microg/mL, respectively, on human promonocytic U-937 cells.


