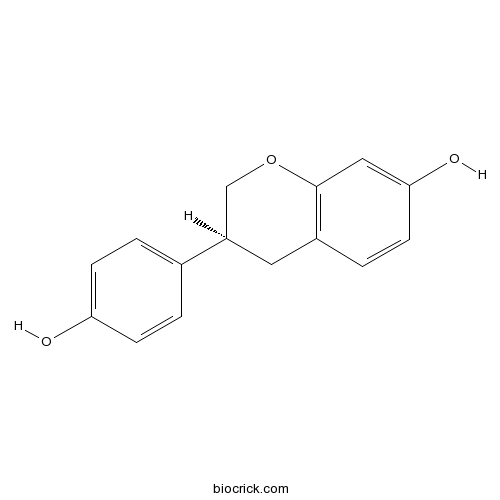
4',7-Isoflavandiolnon-steroidal estrogen CAS# 531-95-3 |
- Ethynodiol diacetate
Catalog No.:BCC4483
CAS No.:297-76-7
- Ethisterone
Catalog No.:BCC4478
CAS No.:434-03-7
- Estriol
Catalog No.:BCN2235
CAS No.:50-27-1
- Erteberel (LY500307)
Catalog No.:BCC4491
CAS No.:533884-09-2
- Medroxyprogesterone acetate
Catalog No.:BCC4485
CAS No.:71-58-9
- Hexestrol
Catalog No.:BCC4484
CAS No.:84-16-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 531-95-3 | SDF | Download SDF |
| PubChem ID | 91469 | Appearance | Powder |
| Formula | C15H14O3 | M.Wt | 242.3 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | DMSO : 100 mg/mL (412.76 mM; Need ultrasonic and warming) | ||
| Chemical Name | (3S)-3-(4-hydroxyphenyl)-3,4-dihydro-2H-chromen-7-ol | ||
| SMILES | C1C(COC2=C1C=CC(=C2)O)C3=CC=C(C=C3)O | ||
| Standard InChIKey | ADFCQWZHKCXPAJ-GFCCVEGCSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 4',7-Isoflavandiol, an estrogen metabolite, affects the ability of soy nuts to improve cardiovascular risk factors. 4',7-Isoflavandiol is a potential anticancer agent against HeLa, with possible mechanisms involved in ROS generation and mitochondrial membrane alteration; it may advance breast cancer potential via up-regulation of the eukaryotic initiation factor 4GI (eIF4GI). |
| Targets | c-Myc | ROS | MMP(e.g.TIMP) | Bcl-2/Bax | Caspase | Estrogen receptor |
| In vitro | Equol induces mitochondria-mediated apoptosis of human cervical cancer cells.[Pubmed: 25202081]Anticancer Res. 2014 Sep;34(9):4985-92.The present study aimed to investigate anticancer properties of 4',7-Isoflavandiol and demonstrate its underlying mechanisms of action in human cervical cancer HeLa cells. |
| In vivo | Effect of soy nuts and equol status on blood pressure, lipids and inflammation in postmenopausal women stratified by metabolic syndrome status.[Pubmed: 25441251]Metabolism. 2015 Feb;64(2):236-43. Soy has been associated with lower risk of cardiovascular disease in Asian countries which consume daily soy. Our study examined whether production of 4',7-Isoflavandiol , an estrogen metabolite, affected the ability of soy nuts to improve cardiovascular risk factors. |
| Kinase Assay | Equol, an isoflavone metabolite, regulates cancer cell viability and protein synthesis initiation via c-Myc and eIF4G.[Pubmed: 25593313]J Biol Chem. 2015 Mar 6;290(10):6047-57.Epidemiological studies implicate dietary soy isoflavones as breast cancer preventives, especially due to their anti-estrogenic properties. However, soy isoflavones may also have a role in promoting breast cancer, which has yet to be clarified. We previously reported that 4',7-Isoflavandiol, a metabolite of the soy isoflavone daidzein, may advance breast cancer potential via up-regulation of the eukaryotic initiation factor 4GI (eIF4GI). In estrogen receptor negative (ER-) metastatic breast cancer cells, equol induced elevated levels of eIF4G, which were associated with increased cell viability and the selective translation of mRNAs that use non-canonical means of initiation, including internal ribosome entry site (IRES), ribosome shunting, and eIF4G enhancers. These mRNAs typically code for oncogenic, survival, and cell stress molecules. Among those mRNAs translationally increased by 4',7-Isoflavandiol was the oncogene and eIF4G enhancer, c-Myc. |

4',7-Isoflavandiol Dilution Calculator

4',7-Isoflavandiol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.1271 mL | 20.6356 mL | 41.2712 mL | 82.5423 mL | 103.1779 mL |
| 5 mM | 0.8254 mL | 4.1271 mL | 8.2542 mL | 16.5085 mL | 20.6356 mL |
| 10 mM | 0.4127 mL | 2.0636 mL | 4.1271 mL | 8.2542 mL | 10.3178 mL |
| 50 mM | 0.0825 mL | 0.4127 mL | 0.8254 mL | 1.6508 mL | 2.0636 mL |
| 100 mM | 0.0413 mL | 0.2064 mL | 0.4127 mL | 0.8254 mL | 1.0318 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
IC50: Not available.
Equol is an isoflavan produced by intestinal bacteria in response to soy isoflavone intake in human. It shows a wide range of activities including antioxidant activity, anti-inflammation activity and anticancer activity. It is reported that Equol specifically binds to 5α-DHT and has a modest affinity for recombinant estrogen receptor ERβ, which may be responsible for most of Equol’s biological properties. [1]
In vitro: In vitro studies were conducted to measure both the binding affinity of Equol for 5alpha-dihydrotestosterone (5alpha-DHT) and the effects of Equol treatment in human prostate cancer (LNCap) cells. It was found that Equol bound to 5alpha-DHT with maximum and half maxim concentrations of 100 nM and 4.8 nM, respectively. In addition, Equol significantly offset the increases in PSA levels from LNCap cells. [1]
In vivo: An in vivo study was performed to investigate effects of equol on rat prostate weight and circulating levels of sex steroid hormones. 1.0 mg/kg of Equol was injected to Long-Evans rats fed with a low isoflavone diet for 25 days. Findings from this study suggested that Equol significantly decreased rat prostate weights and down-regulated serum levels of 5alpha-DHT. However, this agent did not alter levels of LH, testosterone and estradiol. [1]
Clinical trials: A clinical study on hypercholesterolemic patients demonstrated that, after 4 weeks’ dietary intervention with a soy isoflavone-containing food, brachial artery-mediated vasodilatation in equol-producers was notably higher when compared with that in equol-nonproducers. Similar differential effects between equol-producers and nonproducers were aslo reported in arterial stiffness from a study on postmenopausal women taking tibolone. [2]
References:
[1]Lund TD, Blake C, Bu L, Hamaker AN, Lephart ED. iEquol an isoflavonoid: potential for improved prostate health, in vitro and in vivo evidence. Reprod Biol Endocrin. 2011; 9(4): doi: 10.1186/1477-7827-9-4.
[2]Setchell DR and Clerici C. Equol: Pharmacokinetics and biological actions. J Nutr. 2010 Jul; 140(7): 1363S–8S.
- Coumarin-3-Carboxylic Acid
Catalog No.:BCC9220
CAS No.:531-81-7
- Esculin
Catalog No.:BCN5904
CAS No.:531-75-9
- 7-Methoxycoumarin
Catalog No.:BCN2707
CAS No.:531-59-9
- Scopolin
Catalog No.:BCN5701
CAS No.:531-44-2
- Coniferin
Catalog No.:BCN5700
CAS No.:531-29-3
- Androsin
Catalog No.:BCN3842
CAS No.:531-28-2
- Dichotomin
Catalog No.:BCN2836
CAS No.:53093-47-3
- 9,13-Epidioxy-8(14)-abieten-18-oic acid
Catalog No.:BCN1426
CAS No.:5309-35-3
- Morellic acid
Catalog No.:BCN3073
CAS No.:5304-71-2
- Scutebarbatine J
Catalog No.:BCN8134
CAS No.:960302-85-6
- T-5224
Catalog No.:BCC5383
CAS No.:530141-72-1
- Murralongin
Catalog No.:BCN5696
CAS No.:53011-72-6
- Boc-Pyr-OH
Catalog No.:BCC3329
CAS No.:53100-44-0
- Rapamycin (Sirolimus)
Catalog No.:BCC3592
CAS No.:53123-88-9
- Buprenorphine hydrochloride
Catalog No.:BCC5215
CAS No.:53152-21-9
- Euscaphic acid
Catalog No.:BCN5702
CAS No.:53155-25-2
- Delphinidin-3-sambubioside chloride
Catalog No.:BCN3148
CAS No.:53158-73-9
- Acemetacin
Catalog No.:BCC4424
CAS No.:53164-05-9
- Pirfenidone
Catalog No.:BCC5086
CAS No.:53179-13-8
- 6-Aminoindole
Catalog No.:BCC8763
CAS No.:5318-27-4
- Fagomine
Catalog No.:BCC1569
CAS No.:53185-12-9
- Etomidate hydrochloride
Catalog No.:BCC4255
CAS No.:53188-20-8
- Methyocarbamol
Catalog No.:BCC3813
CAS No.:532-03-6
- Anethole trithione
Catalog No.:BCN8510
CAS No.:532-11-6
Effect of soy nuts and equol status on blood pressure, lipids and inflammation in postmenopausal women stratified by metabolic syndrome status.[Pubmed:25441251]
Metabolism. 2015 Feb;64(2):236-43.
OBJECTIVE: Soy has been associated with lower risk of cardiovascular disease in Asian countries which consume daily soy. Our study examined whether production of equol, an estrogen metabolite, affected the ability of soy nuts to improve cardiovascular risk factors. MATERIALS/METHODS: Sixty postmenopausal women participated in a randomized, controlled, crossover trial of a Therapeutic Lifestyle Changes (TLC) diet alone and a TLC diet in which 0.5 cup of soy nuts (25 g of soy protein and 101 mg of aglycone isoflavones) replaced 25 g of nonsoy protein daily. Each diet was followed for 8 weeks at the end of which blood pressure (BP), lipid levels, adhesion molecules and inflammatory markers were measured. RESULTS: Women with MetS had significantly higher baseline body mass index (BMI), BP, triglycerides (TG), and soluble intercellular adhesion molecule (sICAM) than women without MetS. In women with MetS on the soy diet, significant reductions in diastolic BP (7.7%; P=0.02), TG (22.9%; P=0.02), C-reactive protein (CRP) (21.4%; P=0.01) and sICAM (7.3%; P=0.03) were noted among equol producers compared to levels on the TLC diet. No significant changes were noted in equol nonproducers. Similarly, in women without MetS, only equol producers had significant reductions in diastolic BP (3.3%, P=0.02) and CRP (30%, P=0.04). In contrast to women with MetS, TG and sICAM levels were not affected in women without MetS, a finding possibly related to lower baseline levels. CONCLUSIONS: Cardiovascular risk reduction with soy nuts is not uniform and may be greater among producers of equol.
Equol, an isoflavone metabolite, regulates cancer cell viability and protein synthesis initiation via c-Myc and eIF4G.[Pubmed:25593313]
J Biol Chem. 2015 Mar 6;290(10):6047-57.
Epidemiological studies implicate dietary soy isoflavones as breast cancer preventives, especially due to their anti-estrogenic properties. However, soy isoflavones may also have a role in promoting breast cancer, which has yet to be clarified. We previously reported that equol, a metabolite of the soy isoflavone daidzein, may advance breast cancer potential via up-regulation of the eukaryotic initiation factor 4GI (eIF4GI). In estrogen receptor negative (ER-) metastatic breast cancer cells, equol induced elevated levels of eIF4G, which were associated with increased cell viability and the selective translation of mRNAs that use non-canonical means of initiation, including internal ribosome entry site (IRES), ribosome shunting, and eIF4G enhancers. These mRNAs typically code for oncogenic, survival, and cell stress molecules. Among those mRNAs translationally increased by equol was the oncogene and eIF4G enhancer, c-Myc. Here we report that siRNA-mediated knockdown of c-Myc abrogates the increase in cancer cell viability and mammosphere formation by equol, and results in a significant down-regulation of eIF4GI (the major eIF4G isoform), as well as reduces levels of some, but not all, proteins encoded by mRNAs that are translationally stimulated by equol treatment. Knockdown of eIF4GI also markedly reduces an equol-mediated increase in IRES-dependent mRNA translation and the expression of specific oncogenic proteins. However, eIF4GI knockdown did not reciprocally affect c-Myc levels or cell viability. This study therefore implicates c-Myc as a potential regulator of the cancer-promoting effects of equol via up-regulation of eIF4GI and selective initiation of translation on mRNAs that utilize non-canonical initiation, including certain oncogenes.
Equol induces mitochondria-mediated apoptosis of human cervical cancer cells.[Pubmed:25202081]
Anticancer Res. 2014 Sep;34(9):4985-92.
BACKGROUND/AIM: The present study aimed to investigate anticancer properties of equol and demonstrate its underlying mechanisms of action in human cervical cancer HeLa cells. MATERIALS AND METHODS: Inhibition of cell viability was examined by 3-(4,5-dimethylthiazoly-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Apoptosis was evaluated by observation of apoptotic cell morphology, and an increase of annexin-V(+) cells. Western blotting was used to examine apoptosis-related proteins. Flow cytometry was used to measure mitochondrial membrane potential (MMP) and reactive oxygen species (ROS). RESULTS: Equol treatment inhibited HeLa cell proliferation in dose- and time-dependent manner. Equol-induced apoptotic cell death was accompanied by the activation of caspases, and alteration of MMP and mitochondrial membrane proteins; equol also rapidly triggered ROS production. Pre-treatment with N-acetylcysteine blocked loss of MMP, caused increase of Bcl-2-associated X protein (Bax)/B-cell lymphoma 2 (Bcl-2) ratio, caspase-8 activation, and apoptosis induced by equol. CONCLUSION: Equol is a potential anticancer agent against HeLa, with possible mechanisms involved in ROS generation and mitochondrial membrane alteration.


