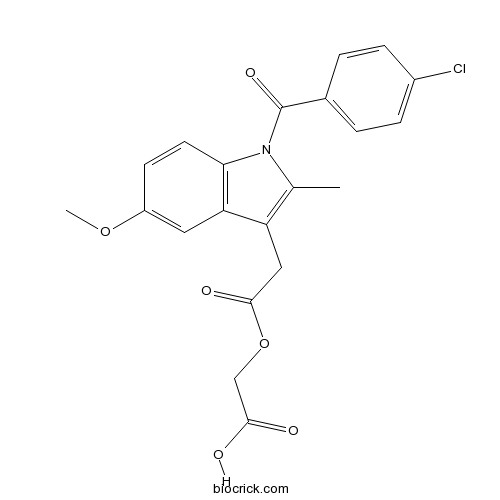
AcemetacinCAS# 53164-05-9 |
- Etifoxine
Catalog No.:BCC1560
CAS No.:21715-46-8
- Etomidate
Catalog No.:BCC1150
CAS No.:33125-97-2
- Etifoxine hydrochloride
Catalog No.:BCC1561
CAS No.:56776-32-0
- Flumazenil
Catalog No.:BCC1259
CAS No.:78755-81-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 53164-05-9 | SDF | Download SDF |
| PubChem ID | 1981 | Appearance | Powder |
| Formula | C21H18ClNO6 | M.Wt | 415.82 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 100 mg/mL (240.49 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) | ||
| Chemical Name | 2-[2-[1-(4-chlorobenzoyl)-5-methoxy-2-methylindol-3-yl]acetyl]oxyacetic acid | ||
| SMILES | CC1=C(C2=C(N1C(=O)C3=CC=C(C=C3)Cl)C=CC(=C2)OC)CC(=O)OCC(=O)O | ||
| Standard InChIKey | FSQKKOOTNAMONP-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C21H18ClNO6/c1-12-16(10-20(26)29-11-19(24)25)17-9-15(28-2)7-8-18(17)23(12)21(27)13-3-5-14(22)6-4-13/h3-9H,10-11H2,1-2H3,(H,24,25) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Acemetacin is a non-steroidal anti-inflammatory drug and a glycolic acid ester of indometacin that is a cyclooxygenase inhibitor.
Target: COX
Acemetacin is a non-steroidal anti-inflammatory drug, used for the treatment of osteoarthritis, rheumatoid arthritis, lower back pain, and relieving post-operative pain. Acemetacin, a glycolic acid ester of indometacin, acts as a prodrug; in the body, it is metabolized to indometacin, which then acts as an inhibitor of cyclooxygenase, producing the anti-inflammatory effects. An advantage of acemetacin is that it reduces gastric damage when compared to indometacin. From Wikipedia. References: | |||||

Acemetacin Dilution Calculator

Acemetacin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.4049 mL | 12.0244 mL | 24.0489 mL | 48.0977 mL | 60.1222 mL |
| 5 mM | 0.481 mL | 2.4049 mL | 4.8098 mL | 9.6195 mL | 12.0244 mL |
| 10 mM | 0.2405 mL | 1.2024 mL | 2.4049 mL | 4.8098 mL | 6.0122 mL |
| 50 mM | 0.0481 mL | 0.2405 mL | 0.481 mL | 0.962 mL | 1.2024 mL |
| 100 mM | 0.024 mL | 0.1202 mL | 0.2405 mL | 0.481 mL | 0.6012 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Acemetacin (Emflex) is a non-steroidal anti-inflammatory drug and a glycolic acid ester of indometacin that is a cyclooxygenase inhibitor.
- Delphinidin-3-sambubioside chloride
Catalog No.:BCN3148
CAS No.:53158-73-9
- Euscaphic acid
Catalog No.:BCN5702
CAS No.:53155-25-2
- Buprenorphine hydrochloride
Catalog No.:BCC5215
CAS No.:53152-21-9
- Rapamycin (Sirolimus)
Catalog No.:BCC3592
CAS No.:53123-88-9
- Boc-Pyr-OH
Catalog No.:BCC3329
CAS No.:53100-44-0
- 4',7-Isoflavandiol
Catalog No.:BCN2855
CAS No.:531-95-3
- Coumarin-3-Carboxylic Acid
Catalog No.:BCC9220
CAS No.:531-81-7
- Esculin
Catalog No.:BCN5904
CAS No.:531-75-9
- 7-Methoxycoumarin
Catalog No.:BCN2707
CAS No.:531-59-9
- Scopolin
Catalog No.:BCN5701
CAS No.:531-44-2
- Coniferin
Catalog No.:BCN5700
CAS No.:531-29-3
- Androsin
Catalog No.:BCN3842
CAS No.:531-28-2
- Pirfenidone
Catalog No.:BCC5086
CAS No.:53179-13-8
- 6-Aminoindole
Catalog No.:BCC8763
CAS No.:5318-27-4
- Fagomine
Catalog No.:BCC1569
CAS No.:53185-12-9
- Etomidate hydrochloride
Catalog No.:BCC4255
CAS No.:53188-20-8
- Methyocarbamol
Catalog No.:BCC3813
CAS No.:532-03-6
- Anethole trithione
Catalog No.:BCN8510
CAS No.:532-11-6
- Tropinone
Catalog No.:BCN1935
CAS No.:532-24-1
- Euparin
Catalog No.:BCN7191
CAS No.:532-48-9
- ar-Turmerone
Catalog No.:BCN7516
CAS No.:532-65-0
- Coixol
Catalog No.:BCN5703
CAS No.:532-91-2
- 2''-O-Galloylhyperin
Catalog No.:BCN1218
CAS No.:53209-27-1
- H-Sar-NH2.HCl
Catalog No.:BCC3333
CAS No.:5325-64-4
Proniosomal oral tablets for controlled delivery and enhanced pharmacokinetic properties of acemetacin.[Pubmed:25319057]
AAPS PharmSciTech. 2015 Apr;16(2):375-83.
Free-flowing proniosomal powders of Acemetacin (AC) were prepared using the slurry method and maltodextrin as carrier. Positively charged proniosomes composed of 70:20:10 of Span 60/cholesterol (Chol)/stearylamine (SA), respectively, were successively compressed into tablets using direct compression method. The tablets were characterized for weight variability, friability, hardness, drug content uniformity, and dissolution properties. The in vivo evaluation of the prepared proniosomes (powder or tablet forms) after oral administration was investigated by the determination of AC and its active metabolite indomethacin (IND) in the blood of albino rabbits. Results indicated that the increase of Chol from 10% to 20% markedly reduced the efflux of the drug. Further Chol addition from 30% to 50% led to increased AC release rates. The proniosome tablets of AC showed greater hardness and disintegration time and less friability than AC plain tablets. The dissolution of proniosomal tablets indicated a lower drug release percentage compared to powdered proniosomes and AC plain tablets. The mean pharmacokinetic parameters of AC and IND from different formulations indicated increased t 1/2 and area under the curve (AUC) of both AC and IND for proniosomal tablets compared with both proniosomal powders and AC plain tablets. This study suggested the formulation of AC proniosomal powder into tablets to control and extend its pharmacologic effects.
Acemetacin-induced fixed drug eruption.[Pubmed:27114641]
Indian J Pharmacol. 2016 Mar-Apr;48(2):219-20.
Fixed drug eruption (FDE) is an adverse effect observed with various drugs such as nonsteroidal anti-inflammatory drugs (NSAIDs) and various antibiotics. Acemetacin, a prodrug of indomethacin, is an NSAID licensed for use in rheumatic disease and other musculoskeletal disorders. We present a case of Acemetacin-induced FDE in a 49-year-old woman. To the best of our knowledge, this is the second case report detailing clinical and histopathological findings of a patient with FDE caused by Acemetacin.
Acemetacin cocrystals and salts: structure solution from powder X-ray data and form selection of the piperazine salt.[Pubmed:25075330]
IUCrJ. 2014 Feb 28;1(Pt 2):136-50.
Acemetacin (ACM) is a non-steroidal anti-inflammatory drug (NSAID), which causes reduced gastric damage compared with indomethacin. However, Acemetacin has a tendency to form a less soluble hydrate in the aqueous medium. We noted difficulties in the preparation of cocrystals and salts of Acemetacin by mechanochemical methods, because this drug tends to form a hydrate during any kind of solution-based processing. With the objective to discover a solid form of Acemetacin that is stable in the aqueous medium, binary adducts were prepared by the melt method to avoid hydration. The coformers/salt formers reported are pyridine carboxamides [nicotinamide (NAM), isonicotinamide (INA), and picolinamide (PAM)], caprolactam (CPR), p-aminobenzoic acid (PABA), and piperazine (PPZ). The structures of an ACM-INA cocrystal and a binary adduct ACM-PABA were solved using single-crystal X-ray diffraction. Other ACM cocrystals, ACM-PAM and ACM-CPR, and the piperazine salt ACM-PPZ were solved from high-resolution powder X-ray diffraction data. The ACM-INA cocrystal is sustained by the acidcdots, three dots, centeredpyridine heterosynthon and N-Hcdots, three dots, centeredO catemer hydrogen bonds involving the amide group. The acidcdots, three dots, centeredamide heterosynthon is present in the ACM-PAM cocrystal, while ACM-CPR contains carboxamide dimers of caprolactam along with acid-carbonyl (ACM) hydrogen bonds. The cocrystals ACM-INA, ACM-PAM and ACM-CPR are three-dimensional isostructural. The carboxylcdots, three dots, centeredcarboxyl synthon in ACM-PABA posed difficulty in assigning the position of the H atom, which may indicate proton disorder. In terms of stability, the salts were found to be relatively stable in pH 7 buffer medium over 24 h, but the cocrystals dissociated to give ACM hydrate during the same time period. The ACM-PPZ salt and ACM-nicotinamide cocrystal dissolve five times faster than the stable hydrate form, whereas the ACM-PABA adduct has 2.5 times faster dissolution rate. The pharmaceutically acceptable piperazine salt of Acemetacin exhibits superior stability, faster dissolution rate and is able to overcome the hydration tendency of the reference drug.
Multivariate optimization of separation conditions for simultaneous determination of acemetacin and chlorzoxazone in a pharmaceutical preparation by HPLC using response surface methodology.[Pubmed:24000743]
J AOAC Int. 2013 Jul-Aug;96(4):723-9.
A new, fast, accurate, precise, and sensitive RP-HPLC method for the simultaneous determination of Acemetacin and chlorzoxazone has been developed. Response surface methodology with a central composite design was used to optimize the acetonitrile and ammonium acetate percentage in the mobile phase and pH of ammonium acetate. The optimum separation was achieved on a C18 column (250 x 4.6 mm id, 5 microm particle size) using the mobile phase methanol-acetonitrile-0.02 M ammonium acetate, pH 9.4 (25 + 35 + 40, v/v/v) at a flow rate of 1.5 mL/min; UV detection at 270 nm, and cyanocobalamin as an internal standard. This developed method was validated and successfully applied to a coated tablet pharmaceutical preparation.


