ar-TurmeroneCAS# 532-65-0 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 532-65-0 | SDF | Download SDF |
| PubChem ID | 160512 | Appearance | Yellow viscous liquid |
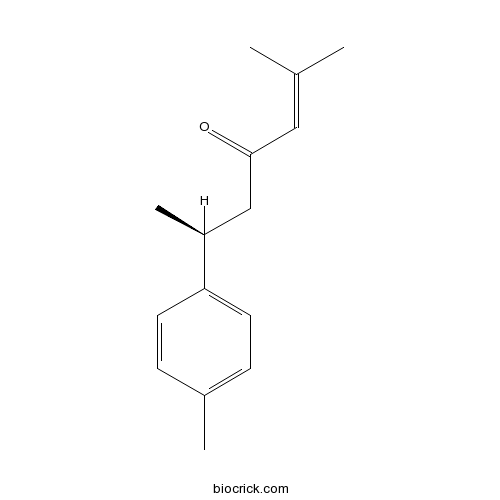
| Formula | C15H20O | M.Wt | 216.32 |
| Type of Compound | Sesquiterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in chloroform, ethanol, methanol and petroleum ether; insoluble in water | ||
| Chemical Name | (6S)-2-methyl-6-(4-methylphenyl)hept-2-en-4-one | ||
| SMILES | CC1=CC=C(C=C1)C(C)CC(=O)C=C(C)C | ||
| Standard InChIKey | NAAJVHHFAXWBOK-ZDUSSCGKSA-N | ||
| Standard InChI | InChI=1S/C15H20O/c1-11(2)9-15(16)10-13(4)14-7-5-12(3)6-8-14/h5-9,13H,10H2,1-4H3/t13-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. ar-Turmerone could be used as a low cost botanical insecticide for integrated management of cabbage looper in vegetable production. 2. ar-Turmerone may have notable antibacterial activity to Bacillus cereus and Staphylococcus aureus, antifungal activity to Aspergillus niger, and cytotoxic activity to Hs 578T (breast tumor) and PC-3 (prostate tumor) cells. 3. ar-Turmerone shows larvicidal and biting deterrent activity against Aedes aegypti and Anopheles quadrimaculatus (Culicidae: Diptera). 4. ar-Turmerone exerts potent anti-inflammatory effects via suppression of the inflammatory cytokines IFN-γ and IL-2. 5. ar-Turmerone induces neural stem cells (NSCs) proliferation, hence it constitutes a promising candidate to support regeneration in neurologic disease. 6. ar-Turmerone displays anticonvulsant properties in both acute seizure models in mice and modulates the expression patterns of two seizure-related genes (c-fos and brain-derived neurotrophic factor [bdnf]) in zebrafish. 7. ar-Turmerone has a cytotoxic effect on L-1210 and HL-60 cells, it also has a repressive effect on P388D1 lymphocytic leukemia. |
| Targets | IFN-γ | IL Receptor | gp120/CD4 | Bcl-2/Bax | Caspase | p53 | p21 |

ar-Turmerone Dilution Calculator

ar-Turmerone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.6228 mL | 23.1139 mL | 46.2278 mL | 92.4556 mL | 115.5695 mL |
| 5 mM | 0.9246 mL | 4.6228 mL | 9.2456 mL | 18.4911 mL | 23.1139 mL |
| 10 mM | 0.4623 mL | 2.3114 mL | 4.6228 mL | 9.2456 mL | 11.557 mL |
| 50 mM | 0.0925 mL | 0.4623 mL | 0.9246 mL | 1.8491 mL | 2.3114 mL |
| 100 mM | 0.0462 mL | 0.2311 mL | 0.4623 mL | 0.9246 mL | 1.1557 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Euparin
Catalog No.:BCN7191
CAS No.:532-48-9
- Tropinone
Catalog No.:BCN1935
CAS No.:532-24-1
- Anethole trithione
Catalog No.:BCN8510
CAS No.:532-11-6
- Methyocarbamol
Catalog No.:BCC3813
CAS No.:532-03-6
- Etomidate hydrochloride
Catalog No.:BCC4255
CAS No.:53188-20-8
- Fagomine
Catalog No.:BCC1569
CAS No.:53185-12-9
- 6-Aminoindole
Catalog No.:BCC8763
CAS No.:5318-27-4
- Pirfenidone
Catalog No.:BCC5086
CAS No.:53179-13-8
- Acemetacin
Catalog No.:BCC4424
CAS No.:53164-05-9
- Delphinidin-3-sambubioside chloride
Catalog No.:BCN3148
CAS No.:53158-73-9
- Euscaphic acid
Catalog No.:BCN5702
CAS No.:53155-25-2
- Buprenorphine hydrochloride
Catalog No.:BCC5215
CAS No.:53152-21-9
- Coixol
Catalog No.:BCN5703
CAS No.:532-91-2
- 2''-O-Galloylhyperin
Catalog No.:BCN1218
CAS No.:53209-27-1
- H-Sar-NH2.HCl
Catalog No.:BCC3333
CAS No.:5325-64-4
- Boc-Tyr(Me)-OH
Catalog No.:BCC3268
CAS No.:53267-93-9
- [Ala11,D-Leu15]-Orexin B
Catalog No.:BCC5877
CAS No.:532932-99-3
- CV 1808
Catalog No.:BCC7163
CAS No.:53296-10-9
- Fmoc-Cys(Bzl)-OH
Catalog No.:BCC3475
CAS No.:53298-33-2
- Tigloidine
Catalog No.:BCN1945
CAS No.:533-08-4
- Sesamol
Catalog No.:BCN2594
CAS No.:533-31-3
- 1,2,4-Benzenetriol
Catalog No.:BCC8409
CAS No.:533-73-3
- L-NMMA acetate
Catalog No.:BCC6788
CAS No.:53308-83-1
- Benzyl carbazate
Catalog No.:BCC8872
CAS No.:5331-43-1
Chemotaxonomic Characterization and in-Vitro Antimicrobial and Cytotoxic Activities of the Leaf Essential Oil of Curcuma longa Grown in Southern Nigeria.[Pubmed:28930216]
Medicines (Basel). 2015 Dec 21;2(4):340-349.
Curcuma longa (turmeric) has been used in Chinese traditional medicine and Ayurvedic medicine for many years. METHODS: The leaf essential oil of C. longa from southern Nigeria was obtained by hydrodistillation and analyzed by gas chromatography-mass spectrometry (GC-MS). The essential oil was screened for in vitro antibacterial, antifungal, and cytotoxic activities. The major components in C. longa leaf oil were ar-Turmerone (63.4%), alpha-turmerone (13.7%), and beta-turmerone (12.6%). A cluster analysis has revealed this to be a new essential oil chemotype of C. longa. The leaf oil showed notable antibacterial activity to Bacillus cereus and Staphylococcus aureus, antifungal activity to Aspergillus niger, and cytotoxic activity to Hs 578T (breast tumor) and PC-3 (prostate tumor) cells. The ar-Turmerone-rich leaf essential oil of C. longa from Nigeria has shown potent biological activity and therapeutic promise.
Insights from zebrafish and mouse models on the activity and safety of ar-turmerone as a potential drug candidate for the treatment of epilepsy.[Pubmed:24349101]
PLoS One. 2013 Dec 13;8(12):e81634.
In a previous study, we uncovered the anticonvulsant properties of turmeric oil and its sesquiterpenoids (ar-Turmerone, alpha-, beta-turmerone and alpha-atlantone) in both zebrafish and mouse models of chemically-induced seizures using pentylenetetrazole (PTZ). In this follow-up study, we aimed at evaluating the anticonvulsant activity of ar-Turmerone further. A more in-depth anticonvulsant evaluation of ar-Turmerone was therefore carried out in the i.v. PTZ and 6-Hz mouse models. The potential toxic effects of ar-Turmerone were evaluated using the beam walking test to assess mouse motor function and balance. In addition, determination of the concentration-time profile of ar-Turmerone was carried out for a more extended evaluation of its bioavailability in the mouse brain. ar-Turmerone displayed anticonvulsant properties in both acute seizure models in mice and modulated the expression patterns of two seizure-related genes (c-fos and brain-derived neurotrophic factor [bdnf]) in zebrafish. Importantly, no effects on motor function and balance were observed in mice after treatment with ar-Turmerone even after administering a dose 500-fold higher than the effective dose in the 6-Hz model. In addition, quantification of its concentration in mouse brains revealed rapid absorption after i.p. administration, capacity to cross the BBB and long-term brain residence. Hence, our results provide additional information on the anticonvulsant properties of ar-Turmerone and support further evaluation towards elucidating its mechanism of action, bioavailability, toxicity and potential clinical application.
Turmeric powder and its derivatives from Curcuma longa rhizomes: Insecticidal effects on cabbage looper and the role of synergists.[Pubmed:27804972]
Sci Rep. 2016 Nov 2;6:34093.
Curcuma longa has well-known insecticidal and repellent effects on insect pests, but its impact on Trichoplusia ni is unknown. In this study, the compound ar-Turmerone, extracted and purified from C. longa rhizomes, was identified, and its insecticidal effects, along with turmeric powder, curcuminoid pigments and crude essential oil were evaluated against this important agricultural pest. The role of natural (sesamol and piperonal) and synthetic [piperonyl butoxide (PBO)] synergists under laboratory and greenhouse conditions were also evaluated. The concentration of ar-Turmerone in C. longa rhizomes harvested was 0.32% (dwt). Turmeric powder and its derivatives caused 10-20% mortality in third instar T. ni at a very low dose (10 mug/larva). Addition of PBO increased toxicity of turmeric powder and its derivatives (90-97% mortality) in most binary combinations (5 mug of turmeric powder or its derivatives +5 mug of PBO), but neither piperonal nor sesamol were active as synergists. The compound ar-Turmerone alone and the combination with PBO reduced larval weight on treated Brassica oleracea in the laboratory and in greenhouse experiments, compared with the negative control. The compound ar-Turmerone could be used as a low cost botanical insecticide for integrated management of cabbage looper in vegetable production.
Suppression of Inflammatory cytokine production by ar-Turmerone isolated from Curcuma phaeocaulis.[Pubmed:25044589]
Chem Biodivers. 2014 Jul;11(7):1034-41.
Rhizomes of Curcuma phaeocaulis Valeton (Zingiberaceae) have traditionally been used for controlling inflammatory conditions. Numerous studies have aimed to isolate and characterize the bioactive constituents of C. phaeocaulis. It has been reported that its anti-inflammatory properties are a result of cyclooxygenase-2 inhibition; however, its effect on the T-cell function remains to be elucidated. In this study, four known sesquiterpenoids, viz., ar-Turmerone (TM), germacrone (GM), (+)-(4S,5S)-germacrone-4,5-epoxide (GE), and curzerenone (CZ), were isolated from C. phaeocaulis rhizomes and evaluated for their effects on the CD4(+) T-cell function. While GM, GE, and CZ had no effect on the activation of splenic T cells or CD4(+) T cells, TM suppressed the interferon (IFN)-gamma production, without affecting the interleukin (IL)-4 expression. TM also decreased the expression of IL-2 in CD4(+) T cells, but did not change their cell-division rates upon stimulation. These results suggest that TM, a major constituent of C. phaeocaulis rhizomes selectively exerts potent anti-inflammatory effects via suppression of the inflammatory cytokines IFN-gamma and IL-2.
Aromatic-turmerone induces neural stem cell proliferation in vitro and in vivo.[Pubmed:25928248]
Stem Cell Res Ther. 2014 Sep 26;5(4):100.
INTRODUCTION: Aromatic (ar-) turmerone is a major bioactive compound of the herb Curcuma longa. It has been suggested that ar-Turmerone inhibits microglia activation, a property that may be useful in treating neurodegenerative disease. Furthermore, the effects of ar-Turmerone on neural stem cells (NSCs) remain to be investigated. METHODS: We exposed primary fetal rat NSCs to various concentrations of ar-Turmerone. Thereafter, cell proliferation and differentiation potential were assessed. In vivo, naive rats were treated with a single intracerebroventricular (i.c.v.) injection of ar-Turmerone. Proliferative activity of endogenous NSCs was assessed in vivo, by using noninvasive positron emission tomography (PET) imaging and the tracer [(18)F]-fluoro-L-thymidine ([(18)F]FLT), as well as ex vivo. RESULTS: In vitro, ar-Turmerone increased dose-dependently the number of cultured NSCs, because of an increase in NSC proliferation (P < 0.01). Proliferation data were supported by qPCR-data for Ki-67 mRNA. In vitro as well as in vivo, ar-Turmerone promoted neuronal differentiation of NSCs. In vivo, after i.c.v. injection of ar-Turmerone, proliferating NSCs were mobilized from the subventricular zone (SVZ) and the hippocampus of adult rats, as demonstrated by both [(18)F]FLT-PET and histology (P < 0.05). CONCLUSIONS: Both in vitro and in vivo data suggest that ar-Turmerone induces NSC proliferation. ar-Turmerone thus constitutes a promising candidate to support regeneration in neurologic disease.
Larvicidal and Biting Deterrent Activity of Essential Oils of Curcuma longa, Ar-turmerone, and Curcuminoids Against Aedes aegypti and Anopheles quadrimaculatus (Culicidae: Diptera).[Pubmed:26336212]
J Med Entomol. 2015 Sep;52(5):979-86.
Essential oils and extract of Curcuma longa, ar-Turmerone, and curcuminoids were evaluated for their larvicidal and deterrent activity against mosquitoes. ar-Turmerone and curcuminoids constituted 36.9, 24.9 and 50.6% of rhizome oil, leaf oil, and rhizome extract, respectively. ar-Turmerone was the major compound of the rhizome oil (36.9%) and leaf oil (24.9%). The ethanolic extract had 15.4% ar-Turmerone with 6.6% bisdesmethoxycurcumin, 6.1% desmethoxycurcumin, and 22.6% curcumin. In in vitro studies, essential oils of the leaf (biting deterrence index [BDI] = 0.98), rhizome (BDI = 0.98), and rhizome ethanolic extract (BDI = 0.96) at 10 microg/cm(2) showed biting deterrent activity similar to DEET at 25 nmol/cm(2) against Aedes aegypti L. Among the pure compounds, ar-Turmerone (BDI = 1.15) showed the biting deterrent activity higher than DEET at 25 nmol/cm(2) whereas the activity of other compounds was lower than DEET. In Anopheles quadrimaculatus Say, only ar-Turmerone showed deterrent activity similar to DEET. In dose-response bioassay, ar-Turmerone showed significantly higher biting deterrence than DEET at all the dosages. ar-Turmerone, at 15 nmol/cm(2), showed activity similar to DEET at 25 nmol/cm(2) and activity at 5 nmol/cm(2) was similar to DEET at 20 and 15 nmol/cm(2). Leaf essential oil with LC(50) values of 1.8 and 8.9 ppm against larvae of An. quadrimaculatus and Ae. aegypti, respectively, showed highest toxicity followed by rhizome oil and ethanolic extract. Among the pure compounds, ar-Turmerone with LC(50) values of 2.8 and 2.5 ppm against larvae of An. quadrimaculatus and Ae. aegypti, respectively, was most toxic followed by bisdesmethoxycurcumin, curcumin, and desmethoxycurcumin.
Immune activation and antitumor response of ar-turmerone on P388D1 lymphoblast cell implanted tumors.[Pubmed:23229920]
Int J Mol Med. 2013 Feb;31(2):386-92.
Aromatic turmerone (ar-Turmerone) has been reported to have a cytotoxic effect on L-1210 and HL-60 cells. In the present study, we investigated the anticancer responses and immune activities in implanted tumor cells. Our study found that ar-Turmerone inhibited the increase in the number of white blood cells, which normally increase by the injection of lymphoblast cells, or P388D1, and ar-Turmerone increased lymphocyte percentage compared to the control. Tumor inhibition rate in the ar-Turmerone-treated group was 11.79%, and the apoptosis indexes of the control, ar-Turmerone and Glivec groups were 4.22+/-1.02, 5.45+/-1.46 and 10.01+/-2.01, respectively, in which only the Glivec-treated group showed a significance. The positive rates of Bcl-2 and Bax proteins which were treated by ar-Turmerone did not show marked differences compared to the control group, but the Bax protein in the Glivec-treated group increased compared to the control group. The density of caspase-1, -3, -6, -9, Bcl-2, Bax, p21 and p53 mRNA in the control, ar-Turmerone and Glivec groups did not change considerably, but the Bax mRNA of the Glivec-treated group increased compared to the control group. The ar-Turmerone-treated group increased T-lymphocyte and B-lymphocyte proliferation activities compared to the control group, which was more significant in T-lymphocyte than in B-lymphocyte proliferation activity. The interleukin-2 (IL2) production activity of the ar-Turmerone group increased compared to the control group. These findings suggest that ar-Turmerone does not have a chemotherapeutic effect on tumor incidence, but it has a repressive effect on P388D1 lymphocytic leukemia. Furthermore, this protective effect of ar-Turmerone from P388D1 lymphocytic leukemia resulted from the increased activity of tumor immunogenicity through increased T-lymphokine production and increased percentage of lymphocytes.


