PirfenidoneTGF-β production inhibitor CAS# 53179-13-8 |
- GSK256066 2,2,2-trifluoroacetic acid
Catalog No.:BCC1605
CAS No.:1415560-64-3
- Nortadalafil
Catalog No.:BCC1806
CAS No.:171596-36-4
- Bay 60-7550
Catalog No.:BCC1405
CAS No.:439083-90-6
- Oglemilast
Catalog No.:BCC1817
CAS No.:778576-62-8
- AN-2728
Catalog No.:BCC1361
CAS No.:906673-24-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 53179-13-8 | SDF | Download SDF |
| PubChem ID | 40632 | Appearance | Powder |
| Formula | C12H11NO | M.Wt | 185.22 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | AMR69 | ||
| Solubility | DMSO : ≥ 100 mg/mL (539.90 mM) *"≥" means soluble, but saturation unknown. | ||
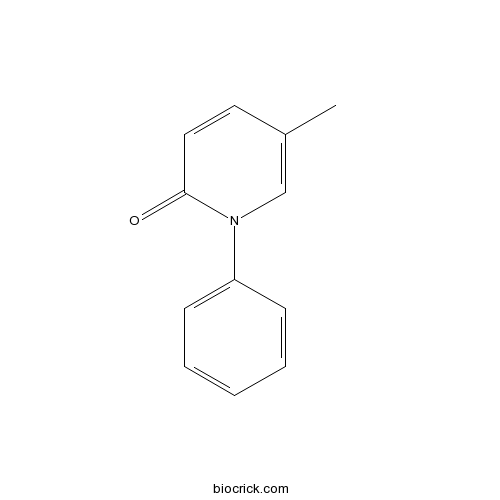
| Chemical Name | 5-methyl-1-phenylpyridin-2-one | ||
| SMILES | CC1=CN(C(=O)C=C1)C2=CC=CC=C2 | ||
| Standard InChIKey | ISWRGOKTTBVCFA-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C12H11NO/c1-10-7-8-12(14)13(9-10)11-5-3-2-4-6-11/h2-9H,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Antifibrotic agent, effective in models of pulmonary and lung fibrosis. Inhibits collagen production and fibroblast proliferation. Regulates cytokine levels following oral administration in vivo. Potent scavenger of free radicals and inhibitor of lipid peroxidation. |

Pirfenidone Dilution Calculator

Pirfenidone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.399 mL | 26.9949 mL | 53.9898 mL | 107.9797 mL | 134.9746 mL |
| 5 mM | 1.0798 mL | 5.399 mL | 10.798 mL | 21.5959 mL | 26.9949 mL |
| 10 mM | 0.5399 mL | 2.6995 mL | 5.399 mL | 10.798 mL | 13.4975 mL |
| 50 mM | 0.108 mL | 0.5399 mL | 1.0798 mL | 2.1596 mL | 2.6995 mL |
| 100 mM | 0.054 mL | 0.2699 mL | 0.5399 mL | 1.0798 mL | 1.3497 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Pirfenidone is an inhibitor for TGF-β production and TGF-β stimulated collagen production, reduces production of TNF-α and IL-1β, and also has anti-fibrotic and anti-inflammatory properties. Phase 3.
- Acemetacin
Catalog No.:BCC4424
CAS No.:53164-05-9
- Delphinidin-3-sambubioside chloride
Catalog No.:BCN3148
CAS No.:53158-73-9
- Euscaphic acid
Catalog No.:BCN5702
CAS No.:53155-25-2
- Buprenorphine hydrochloride
Catalog No.:BCC5215
CAS No.:53152-21-9
- Rapamycin (Sirolimus)
Catalog No.:BCC3592
CAS No.:53123-88-9
- Boc-Pyr-OH
Catalog No.:BCC3329
CAS No.:53100-44-0
- 4',7-Isoflavandiol
Catalog No.:BCN2855
CAS No.:531-95-3
- Coumarin-3-Carboxylic Acid
Catalog No.:BCC9220
CAS No.:531-81-7
- Esculin
Catalog No.:BCN5904
CAS No.:531-75-9
- 7-Methoxycoumarin
Catalog No.:BCN2707
CAS No.:531-59-9
- Scopolin
Catalog No.:BCN5701
CAS No.:531-44-2
- Coniferin
Catalog No.:BCN5700
CAS No.:531-29-3
- 6-Aminoindole
Catalog No.:BCC8763
CAS No.:5318-27-4
- Fagomine
Catalog No.:BCC1569
CAS No.:53185-12-9
- Etomidate hydrochloride
Catalog No.:BCC4255
CAS No.:53188-20-8
- Methyocarbamol
Catalog No.:BCC3813
CAS No.:532-03-6
- Anethole trithione
Catalog No.:BCN8510
CAS No.:532-11-6
- Tropinone
Catalog No.:BCN1935
CAS No.:532-24-1
- Euparin
Catalog No.:BCN7191
CAS No.:532-48-9
- ar-Turmerone
Catalog No.:BCN7516
CAS No.:532-65-0
- Coixol
Catalog No.:BCN5703
CAS No.:532-91-2
- 2''-O-Galloylhyperin
Catalog No.:BCN1218
CAS No.:53209-27-1
- H-Sar-NH2.HCl
Catalog No.:BCC3333
CAS No.:5325-64-4
- Boc-Tyr(Me)-OH
Catalog No.:BCC3268
CAS No.:53267-93-9
Antifibrotic effect of pirfenidone in a mouse model of human nonalcoholic steatohepatitis.[Pubmed:28303974]
Sci Rep. 2017 Mar 17;7:44754.
Non-alcoholic steatohepatitis (NASH) is characterized by steatosis with lobular inflammation and hepatocyte injury. Pirfenidone (PFD) is an orally bioavailable pyridone derivative that has been clinically used for the treatment of idiopathic pulmonary fibrosis. However, it remains unknown whether PFD improves liver fibrosis in a mouse model with human NASH-like phenotypes. In this study, we employed melanocortin 4 receptor-deficient (MC4R-KO) mice as a mouse model with human NASH-like phenotypes to elucidate the effect and action mechanisms of PFD on the development of NASH. PFD markedly attenuated liver fibrosis in western diet (WD)-fed MC4R-KO mice without affecting metabolic profiles or steatosis. PFD prevented liver injury and fibrosis associated with decreased apoptosis of liver cells in WD-fed MC4R-KO mice. Pretreatment of PFD inhibited the tumor necrosis factor-alpha (TNF-alpha)-induced liver injury and fibrogenic responses associated with decreased apoptosis of liver cells in wild-type mice. PFD also prevented TNF-alpha-induced hepatocyte apoptosis in vitro with reduced activation of caspase-8 and -3. This study provides evidence for the antifibrotic effect of PFD in a mouse model of human NASH. The data of this study highlight hepatocyte apoptosis as a potential therapeutic target, and suggest that PFD can be repositioned as an antifibrotic drug for human NASH.
Predicting Life Expectancy for Pirfenidone in Idiopathic Pulmonary Fibrosis.[Pubmed:28287347]
J Manag Care Spec Pharm. 2017 Mar;23(3-b Suppl):S17-S24.
BACKGROUND: Conducting an adequately powered survival study in idiopathic pulmonary fibrosis (IPF) is challenging due to the rare nature of the disease and the need for extended follow-up. Consequently, registration trials of IPF treatments have not been designed to estimate long-term survival. OBJECTIVE: To predict life expectancy for patients with IPF receiving Pirfenidone versus best supportive care (BSC) in a population that met the inclusion criteria of patients enrolled in the ASCEND and CAPACITY trials. METHODS: Kaplan-Meier survival data for Pirfenidone and BSC were obtained from randomized controlled clinical studies (CAPACITY, ASCEND), an open-label extension study (RECAP), and the Inova Fairfax Hospital database. Data from the Inova registry were matched to the inclusion criteria of the CAPACITY and ASCEND trials. Life expectancy was estimated by the area under the curve of parametric survival distributions fit to the Kaplan-Meier data. RESULTS: Mean (95% confidence interval) life expectancy was calculated as 8.72 (7.65-10.15) years with Pirfenidone and 6.24 (5.38-7.18) years with BSC. Therefore, Pirfenidone improved life expectancy by 2.47 (1.26-4.17) years compared with BSC. In addition, treatment with Pirfenidone recuperated 25% of the expected years of life lost due to IPF. Sensitivity analyses found that results were sensitive to the choice of parametric survival distribution, and alternative piecewise and parametric approaches. CONCLUSIONS: This analysis suggests that this population of patients with IPF has an improved life expectancy if treated with Pirfenidone compared with BSC. DISCLOSURES: This study was funded by InterMune International AG, a wholly owned Roche subsidiary since 2014. Fisher was previously employed by InterMune UK, a wholly owned Roche subsidiary, until July 2015. He is currently employed by FIECON, which has received funding from F. Hoffmann-La Roche for consulting services. Nathan has received consulting fees from Roche-Genentech and Boehringer Ingelheim. He is also on the speakers' bureau for Roche-Genentech and Boehringer Ingelheim and has received research funding from both companies. Hill was previously employed by InterMune UK until October 2014. Hill and Marshall are employees of MAP BioPharma, which has received funding from F. Hoffmann-La Roche for consulting services. Dejonckheere and Thuresson are employees of F. Hoffmann-La Roche. Maher has received grants, consulting fees, and speaker fees from GlaxoSmithKline and UCB, and grants from Novartis. He has also received consulting fees and speaker fees from AstraZeneca, Bayer, Biogen Idec, Boehringer Ingelheim, Cipla, Lanthio, InterMune International AG, F. Hoffmann-La Roche, Sanofi-Aventis, and Takeda. Maher is supported by a National Institute for Health Research Clinician Scientist Fellowship (NIHR Ref: CS: -2013-13-017). Study concept and design were contributed by Fisher, Hill, Marshall, and Dejonckheere. Fisher, Nathan, and Thuresson collected the data, along with Hill and Marshall. Data interpretation was performed by Fisher, Maher, Nathan, and Dejonckheere. The manuscript was written primarily by Fisher, along with Maher and Dejonckheere, and revised by Fisher and Maher, along with the other authors.
Pirfenidone and nintedanib for pulmonary fibrosis in clinical practice: Tolerability and adverse drug reactions.[Pubmed:28317233]
Respirology. 2017 Aug;22(6):1171-1178.
BACKGROUND AND OBJECTIVE: The real-world tolerability of Pirfenidone and nintedanib in non-clinical trial patients is unknown. Many patients with pulmonary fibrosis have significant medical co-morbidities or baseline characteristics that exclude them from clinical trial participation. METHODS: We conducted a retrospective chart review study on subjects prescribed nintedanib or Pirfenidone for pulmonary fibrosis treatment (any aetiology) from September 2014 to February 2016. A total of 186 subjects were included: 129 received Pirfenidone and 57 were prescribed nintedanib and followed up for mean observation periods of 52 +/- 17 weeks for Pirfenidone and 41 +/- 15 weeks for nintedanib. The primary outcome was drug discontinuation as a result of an adverse event. RESULTS: Subjects had significant respiratory impairment at baseline, 63% required home oxygen therapy and mean diffusion capacity of carbon monoxide (DLCO) was 36 +/- 14% predicted. Drug discontinuation as a result of an adverse event occurred in 20.9% of subjects on Pirfenidone and 26.3% on nintedanib. Drug discontinuation rates for both Pirfenidone and nintedanib did not significantly differ from corresponding large clinical trials (ASCEND/CAPACITY and INPULSIS 1 and 2, respectively). Adverse events that occurred with highest frequency on Pirfenidone were nausea (26.4%), rash/photosensitivity (14.7%) and dyspepsia/gastroesophageal reflux disease (GERD) (12.4%). Diarrhoea (52.6%) and nausea (29.8%) were reported most often with nintedanib therapy. CONCLUSION: Patients with pulmonary fibrosis treated with nintedanib or Pirfenidone in routine clinical practice had drug tolerability and adverse event profiles comparable with subjects enrolled in clinical trials despite having a greater degree of respiratory impairment and a high prevalence of co-morbid medical conditions.
Pirfenidone inhibits post-traumatic proliferative vitreoretinopathy.[Pubmed:28304388]
Eye (Lond). 2017 Sep;31(9):1317-1328.
PurposeThe purpose of the study was to evaluate the efficacy and safety of intravitreal Pirfenidone for inhibition of proliferative vitreoretinopathy (PVR) in a model of penetrating ocular injury.Patients and methodsPenetrating trauma was induced on the retina of rabbit and treated either with 0.1 ml of phosphate-buffered saline (PBS) or 0.1 ml of 0.5% Pirfenidone, and development of PVR was evaluated clinically and graded after 1 month. Histopathology and immunohistochemistry with transforming growth factor beta (TGFbeta), alpha smooth muscle actin (alphaSMA), and collagen-1 were performed to assess the fibrotic changes. Expression of cytokines in the vitro-retinal tissues at different time points following Pirfenidone and PBS injection was examined by RT-PCR. Availability of Pirfenidone in the vitreous of rabbit at various time points was determined by high-performance liquid chromatography following injection of 0.1 ml of 0.5% Pirfenidone. In normal rabbit eye, 0.1 ml of 0.5% Pirfenidone was injected to evaluate any toxic effect.ResultsClinical assessment and grading revealed prevention of PVR formation in Pirfenidone-treated animals, gross histology, and histopathology confirmed the observation. Immunohistochemistry showed prevention in the expression of collagen-I, alphaSMA, and TGFbeta in the Pirfenidone-treated eyes compared to the PBS-treated eyes. Pirfenidone inhibited increased gene expression of cytokines observed in control eyes. Pirfenidone could be detected up to 48 h in the vitreous of rabbit eye following single intravitreal injection. Pirfenidone did not show any adverse effect following intravitreal injection; eyes were devoid of any abnormal clinical sign, intraocular pressure, and electroretinography did not show any significant change and histology of retina remained unchanged.ConclusionThis animal study shows that Pirfenidone might be a potential therapy for PVR. Further clinical study will be useful to evaluate the clinical application of Pirfenidone.
Protection of pirfenidone against an early phase of oleic acid-induced acute lung injury in rats.[Pubmed:15608079]
J Pharmacol Exp Ther. 2005 Apr;313(1):379-88.
The potential role of PFD [5-methyl-L-phenyl-2-(1H)-pyridone], an antifibrotic compound with anti-inflammatory effects, in several models of acute lung injury (ALI) has gained increasing attention; however, the protective effect of PFD in oleic acid (OA)-induced ALI remains unknown. We hypothesized that PFD protects from OA-induced ALI in rats, and we hoped to obtain the optimum preclinical conditions with PFD in ALI. Sprague-Dawley rats were randomized into five groups (five rats per group): normal control group, OA-treated group (0.15 ml/kg), and three PFD-treated groups (20, 40, and 80 mg/kg p.o., respectively). Arterial blood gases, lung wet/dry weight ratio, and postmortem histological changes were determined 0.5, 1, 2, 6, and 24 h after OA challenge. Electron spin resonance spectroscopy was used for free radical detection and measurement. Experiments were examined based on the orthogonal test L4 (4(2)) setting two factors (PFD dose and PFD valid time) with four different levels. The results of the orthogonal test showed that the sequence of effect of PFD was 0.5 h (oxygen radicals), 1 h (histological changes), 2 h (lung edema), and 6 h (partial pressure of oxygen) after OA challenge, and 40 mg/kg PFD was the most effective dose in this study. We conclude that PFD protects against OA-induced ALI in rats. The mechanism of these protective effects partly involves decrease of oxygen radicals. The data of this study proves that the orthogonal test will be a powerful method to help obtain the optimum experimental conditions with PFD in ALI in the future.
Effects of pirfenidone on procollagen gene expression at the transcriptional level in bleomycin hamster model of lung fibrosis.[Pubmed:10087006]
J Pharmacol Exp Ther. 1999 Apr;289(1):211-8.
A time course study was carried out to elucidate the mechanisms for antifibrotic effect of Pirfenidone (PD). Hamsters were intratracheally (i.t.) instilled with saline (SA) or bleomycin (BL) (7.5 units/kg/5 ml). The animals were fed a diet containing 0.5% PD or the same control diet (CD) without the drug 2 days before and throughout the study. The animals were sacrificed at various times after instillation. The lung hydroxyproline level in BL + CD groups was gradually increased and peaked at 21 days to 181% of the SA + CD control. The BL + PD-treated groups showed a gradual decrease in their lung collagen content, showing a maximum reduction of 40% at day 21. The lung malondialdehyde levels of the BL + CD groups were increased by several-fold of the corresponding SA + CD groups at various times. The lung prolyl hydroxylase (PH) activities in the BL + CD groups were also increased by several-fold of the corresponding SA + CD groups at these time points. The hamsters in the BL + PD showed a gradual decrease in the lung malondialdehyde levels from 10 to 21days compared with their corresponding BL + CD groups. Treatment with PD also reduced the lung PH activities in the BL + PD groups compared with the corresponding BL + CD groups. However, PD failed to manifest any direct inhibitory effect on PH activity in vitro. BL treatment increased the lung procollagen I and III gene expressions in the BL + CD groups by several-fold at varying times compared with the corresponding SA + CD, and treatment with PD in the BL + PD groups significantly down-regulated the BL-induced overexpression of these genes. Studies evaluating the regulation of these genes at the transcriptional level revealed PD significantly reduced the transcription of PC I at 14 days. Our results indicate that the antifibrotic effect of PD was partly due to suppression of the BL-induced inflammatory events and partly due to down-regulation of BL-induced overexpression of lung procollagen I and III genes.
Pirfenidone diminishes cyclophosphamide-induced lung fibrosis in mice.[Pubmed:9067480]
Toxicol Lett. 1997 Feb 7;90(2-3):125-32.
The deposition of excess or abnormal collagen characteristic of pulmonary fibrosis can disrupt gas exchange resulting in severe respiratory impairment. There currently are no effective pharmacologic agents available that inhibit the fibrotic process. Pirfenidone (5-methyl-1-phenyl-2-(1H)-pyridone) is an investigational drug that, when administered at 0.5% (w/w) of the diet, decreases both histologic and biochemical evidence of lung fibrosis in hamsters treated intratracheally with bleomycin. The effectiveness of Pirfenidone against lung fibrosis initiated by a systemically administered agent was investigated in mice treated intraperitoneally with 200 mg/kg cyclophosphamide (CP). Control and treated animals were fed a diet containing 0.277% (w/w) Pirfenidone beginning 1 day after CP. Despite anorexia in the CP-treated mice the first day after treatment, they ingested a greater average Pirfenidone dose over 20 days than saline-treated control mice (717 +/- 44 versus 564 +/- 30 mg/kg per day, respectively). Total lung hydroxyproline content, an index of fibrosis, was significantly lower 21 days after treatment with CP plus Pirfenidone as compared to mice treated with CP alone. Although microscopic lung fibrosis scores were not significantly decreased by Pirfenidone in CP-treated mice, the overall incidence of fibrosis was significantly decreased. Histologically, mice treated with CP showed fibrosis while mice treated with CP plus Pirfenidone exhibited fewer abnormalities. The rate of hydroxyproline synthesis by lung tissue 9 days after treatment with CP was significantly elevated. This rate was not affected by Pirfenidone treatment. Overall, these data support an antifibrotic effect of Pirfenidone against CP-induced lung fibrosis in mice. The mechanism of its effect is not known, but appears to be unrelated to an inhibition of collagen synthesis.


