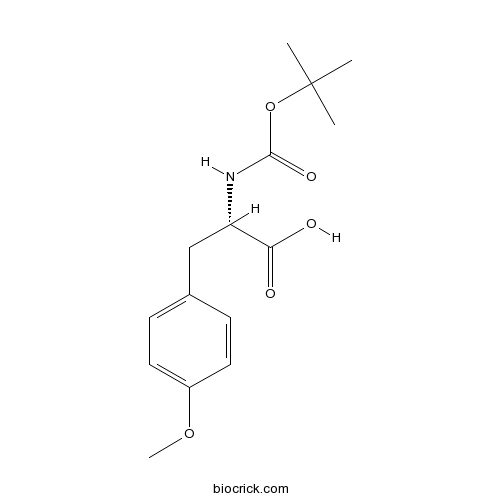
Boc-Tyr(Me)-OHCAS# 53267-93-9 |
- Cisplatin
Catalog No.:BCN1552
CAS No.:14283-03-5
- Z-VAD-FMK
Catalog No.:BCC1126
CAS No.:187389-52-2
- Z-WEHD-FMK
Catalog No.:BCC1139
CAS No.:210345-00-9
- Z-LEHD-FMK
Catalog No.:BCC5117
CAS No.:210345-04-3
- AZ 10417808
Catalog No.:BCC2356
CAS No.:331645-84-2
- Apoptosis Activator 2
Catalog No.:BCC2099
CAS No.:79183-19-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 53267-93-9 | SDF | Download SDF |
| PubChem ID | 2762280 | Appearance | Powder |
| Formula | C15H21NO5 | M.Wt | 295.3 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (2S)-3-(4-methoxyphenyl)-2-[(2-methylpropan-2-yl)oxycarbonylamino]propanoic acid | ||
| SMILES | CC(C)(C)OC(=O)NC(CC1=CC=C(C=C1)OC)C(=O)O | ||
| Standard InChIKey | SLWWWZWJISHVOU-LBPRGKRZSA-N | ||
| Standard InChI | InChI=1S/C15H21NO5/c1-15(2,3)21-14(19)16-12(13(17)18)9-10-5-7-11(20-4)8-6-10/h5-8,12H,9H2,1-4H3,(H,16,19)(H,17,18)/t12-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Boc-Tyr(Me)-OH Dilution Calculator

Boc-Tyr(Me)-OH Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.3864 mL | 16.9319 mL | 33.8639 mL | 67.7277 mL | 84.6597 mL |
| 5 mM | 0.6773 mL | 3.3864 mL | 6.7728 mL | 13.5455 mL | 16.9319 mL |
| 10 mM | 0.3386 mL | 1.6932 mL | 3.3864 mL | 6.7728 mL | 8.466 mL |
| 50 mM | 0.0677 mL | 0.3386 mL | 0.6773 mL | 1.3546 mL | 1.6932 mL |
| 100 mM | 0.0339 mL | 0.1693 mL | 0.3386 mL | 0.6773 mL | 0.8466 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Boc-Tyr(Me)-OH
- H-Sar-NH2.HCl
Catalog No.:BCC3333
CAS No.:5325-64-4
- 2''-O-Galloylhyperin
Catalog No.:BCN1218
CAS No.:53209-27-1
- Coixol
Catalog No.:BCN5703
CAS No.:532-91-2
- ar-Turmerone
Catalog No.:BCN7516
CAS No.:532-65-0
- Euparin
Catalog No.:BCN7191
CAS No.:532-48-9
- Tropinone
Catalog No.:BCN1935
CAS No.:532-24-1
- Anethole trithione
Catalog No.:BCN8510
CAS No.:532-11-6
- Methyocarbamol
Catalog No.:BCC3813
CAS No.:532-03-6
- Etomidate hydrochloride
Catalog No.:BCC4255
CAS No.:53188-20-8
- Fagomine
Catalog No.:BCC1569
CAS No.:53185-12-9
- 6-Aminoindole
Catalog No.:BCC8763
CAS No.:5318-27-4
- Pirfenidone
Catalog No.:BCC5086
CAS No.:53179-13-8
- [Ala11,D-Leu15]-Orexin B
Catalog No.:BCC5877
CAS No.:532932-99-3
- CV 1808
Catalog No.:BCC7163
CAS No.:53296-10-9
- Fmoc-Cys(Bzl)-OH
Catalog No.:BCC3475
CAS No.:53298-33-2
- Tigloidine
Catalog No.:BCN1945
CAS No.:533-08-4
- Sesamol
Catalog No.:BCN2594
CAS No.:533-31-3
- 1,2,4-Benzenetriol
Catalog No.:BCC8409
CAS No.:533-73-3
- L-NMMA acetate
Catalog No.:BCC6788
CAS No.:53308-83-1
- Benzyl carbazate
Catalog No.:BCC8872
CAS No.:5331-43-1
- Isogosferol
Catalog No.:BCN5704
CAS No.:53319-52-1
- PP 3
Catalog No.:BCC7486
CAS No.:5334-30-5
- Z-Leu-OH.DCHA
Catalog No.:BCC2765
CAS No.:53363-87-4
- Boc-N-Me-Leu-OH
Catalog No.:BCC2616
CAS No.:53363-89-6
Cyclic morphiceptin analogs: cyclization studies and opioid activities in vitro.[Pubmed:8985782]
Int J Pept Protein Res. 1996 Dec;48(6):495-502.
Attempts were undertaken to develop cyclic beta-casomorphin-5 analogs with improved opioid activity profiles by deletion of the glycine residue in position 5, leading to analogs structurally related to the opioid peptide morphiceptin (H-Tyr-Pro-Phe-Pro-NH2). The tetrapeptide sequence Boc-Tyr(tBu)-D-Xaa-Phe-Yaa-OH (Xaa = Lys, Orn, A2bu; Yaa = Pro in L- or D-configuration) was used to study the influence of ring size and chirality on the yield of cyclization between the omega-amino group of Xaa and the C-terminal carboxyl group. In all cases the cyclization reaction was performed under identical experimental conditions to allow a direct comparison with regard to yield and homogeneity. The reaction products were purified by crystallization and liquid chromatography, and were characterized by HPLC, TLC, electrospray mass spectrometry and 1H-NMR spectroscopy. In none of the reactions performed with the cyclization precursors containing proline in the L-configuration could a cyclic monomer be detected, and the cyclodimer (7-9) was the exclusive product in each case. Cyclodimerization was also the favored reaction in the attempted formation of the 11-membered ring of the cyclic [D-A2bu2, D-Pro4]-morphiceptin analog 12, since only traces of the monomer were found. In the case of both the [D-Lys2, D-Pro4]-analog 10 and the [D-Orn2, D-Pro4]-analog 11, the cyclomonomer/cyclodimer ratio was about 80:20. The cyclic monomers 10 and 11 showed high opioid activity in the mu-receptor-representative guinea pig ileum assay (IC50 = 2-5 nM) and in the delta-receptor representative mouse vas deferens assay (IC50 = 50-60 nM), whereas the potency of the cyclodimers was 2-3 orders of magnitude lower in both in vitro bioassays.
Amino acids and peptides. LII. Design and synthesis of opioid mimetics containing a pyrazinone ring and examination of their opioid receptor binding activity.[Pubmed:9775433]
Chem Pharm Bull (Tokyo). 1998 Sep;46(9):1374-82.
An amino group was introduced to the 3 or 6 position of a pyrazinone ring by cyclization of dipeptidyl chloromethyl ketones. Boc-Tyr-OH was coupled with the amino function, followed by removal of the Boc group to give pyrazinone ring-containing tyrosine derivatives. Of the various tyrosine derivatives prepared, 5-methyl-6-beta-phenethyl-3-tyrosylaminobutyl-2(1H)-pyrazinone exhibited strong binding to the mu-opioid receptor with a Ki value of 55.8 nM and to the delta-opioid receptor with a Ki value of 2165 nM and with a Ki mu/Ki delta value of 0.026.
Delta opioidmimetic antagonists: prototypes for designing a new generation of ultraselective opioid peptides.[Pubmed:8529134]
Mol Med. 1995 Sep;1(6):678-89.
BACKGROUND: Tyr-Tic (1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid) and Tyr-Tic-Ala were the first peptides with delta opioid antagonist activity lacking Phe, considered essential for opioid activity based on the N-terminal tripeptide sequence (Tyr-D-Xaa-Phe) of amphibian skin opioids. Analogs were then designed to restrain the rotational flexibility of Tyr by the substitution of 2,6-dimethyl-L-tyrosine (Dmt). MATERIALS AND METHODS: Tyr and Dmt peptides were synthesized by solid phase and solution methods using Fmoc technology or condensing Boc-Dmt-OH or Boc-Tyr(But)-OH with H-L-Tic-OBut or H-D-Tic-OBut, respectively. Peptides were purified (> 99%) by HPLC and characteristics determined by 1H-NMR, FAB-MS, melting point, TLC, and amino acid analyses. RESULTS: H-Dmt-Tic-OH had high affinity (Ki delta = 0.022 nM) and extraordinary selectivity (Ki mu/Ki delta = 150,000); H-Dmt-Tic-Ala-OH had a Ki delta = 0.29 nM and delta selectivity = 20,000. Affinity and selectivity increased 8700- and 1000-fold relative to H-Tyr-Tic-OH, respectively. H-Dmt-Tic-OH and H-Dmt-Tic-NH2 fitted one-site receptor binding models (eta = 0.939-0.987), while H-Dmt-Tic-ol, H-Dmt-Tic-Ala-OH and H-Dmt-Tic-Ala-NH2 best fitted two-site models (eta = 0.708-0.801, F 18.9-26.0, p < 0.0001). Amidation increased mu affinity by 10- to 100-fold and acted synergistically with D-Tic2 to reverse selectivity (delta-->mu). Dmt-Tic di- and tripeptides exhibited delta antagonist bioactivity (Ke = 4-66 nM) with mouse vas deferens and lacked agonist mu activity (> 10 microM) in guinea-pig ileum preparations. Dmt-Tic analogs weakly interacted with kappa receptors in the 1 to > 20 microM range. CONCLUSIONS: Dmt-Tic opioidmimetic peptides represent a highly potent class of opioid peptide antagonists with greater potency than the nonopioid delta antagonist naltrindole and have potential application as clinical and therapeutic compounds.
The synthesis, distribution, and anti-hepatic cancer activity of YSL.[Pubmed:15336278]
Bioorg Med Chem. 2004 Sep 15;12(18):4989-94.
YSL was prepared stepwise from C terminal to N terminal with the side chain un-protective amino acids, Boc-Leu-OMe, Boc-Ser-OH, and Boc-Tyr-OH, as the starting materials in 39.5% total yield (31.2g/per batch). With the side chain un-protective Boc-(3,5-dibromo)-Tyr-OH and HCl.Ser-Leu-OMe as the starting materials (3,5-(3)H-Tyr)-Ser-Leu-OH was obtained in 29% yield. The determination of radioactive quantity in the urine and feces indicated that even after the administration for 130 h only 8.4% (5.35% in urine and 3.05% in feces) of total radioactive quantity from the metabolite of [3,5-(3)H-Tyr]-Ser-Leu-OH were monitored. The distribution study revealed the relative accumulation level of the individual tissue was arranged in the sequence of spleen>liver>kidney>lung>heart>muscle>brain. Selecting hepatic cancer as the target YSL significantly increased the survival time of H22 tumor cells implanted mice.
Synthesis and antinociceptive activity of [D-Met2, Pro5] enkephalin [N1,5-beta-D-2,3,4,6-O-tetraacetylglycosyl]--amide and [D-Met2, Pro5] enkephalinamide.[Pubmed:9342551]
Drug Des Discov. 1997 Aug;15(2):83-94.
Tetra-O-acetylgalactopyranosylamine and tetra-O-acetylglucopyranosylamine of D-Met2, Pro5 enkephalin were designed and synthesized to enhance their membrane penetration, biological activity and resistance to proteolytic hydrolysis. Three approaches to the synthesis were attempted, which lead to a new synthetic scheme with a higher yield and enhanced ease of purification. The improved procedure involved attaching the tetra-O-acetylglycopyranosylamine to a t-Boc-Gly-Phe-Pro-OH peptide, removing the t-Boc, and condensing it with t-Boc-Tyr-D-Met-OH. Biological evaluation in vivo showed that these acetylglycopyranosylamine derivatives bind to mu and delta opioid receptors in homogenate binding assays and possess analgesic activity. The analgesic potency was less than that of the parent compound D-Met2, Pro5 enkephalin. These acetylglycopyranosylamine derivatives showed enhanced lipophilicity compared to their parent compound by a partition coefficient study and they also showed greater membrane permeability, using the rabbit cornea as a model system. These derivatives also are resistant to hydrolytic enzymes as compared to the endogenous met-enkephalin when evaluated in homogenized iris-ciliary body and aqueous humor from rabbit eyes.


