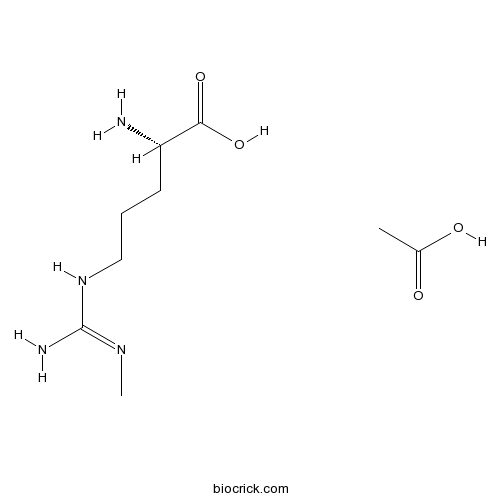
L-NMMA acetateCAS# 53308-83-1 |
- GSK1904529A
Catalog No.:BCC1062
CAS No.:1089283-49-7
- PQ 401
Catalog No.:BCC1159
CAS No.:196868-63-0
- BMS-536924
Catalog No.:BCC1177
CAS No.:468740-43-4
- NVP-ADW742
Catalog No.:BCC4553
CAS No.:475488-23-4
- AG-1024
Catalog No.:BCC1242
CAS No.:65678-07-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 53308-83-1 | SDF | Download SDF |
| PubChem ID | 135242 | Appearance | Powder |
| Formula | C9H20N4O4 | M.Wt | 248.28 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | H2O : ≥ 50 mg/mL (201.39 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | acetic acid;(2S)-2-amino-5-[(N'-methylcarbamimidoyl)amino]pentanoic acid | ||
| SMILES | CC(=O)O.CN=C(N)NCCCC(C(=O)O)N | ||
| Standard InChIKey | IKPNWIGTWUZCKM-JEDNCBNOSA-N | ||
| Standard InChI | InChI=1S/C7H16N4O2.C2H4O2/c1-10-7(9)11-4-2-3-5(8)6(12)13;1-2(3)4/h5H,2-4,8H2,1H3,(H,12,13)(H3,9,10,11);1H3,(H,3,4)/t5-;/m0./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

L-NMMA acetate Dilution Calculator

L-NMMA acetate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.0277 mL | 20.1386 mL | 40.2771 mL | 80.5542 mL | 100.6928 mL |
| 5 mM | 0.8055 mL | 4.0277 mL | 8.0554 mL | 16.1108 mL | 20.1386 mL |
| 10 mM | 0.4028 mL | 2.0139 mL | 4.0277 mL | 8.0554 mL | 10.0693 mL |
| 50 mM | 0.0806 mL | 0.4028 mL | 0.8055 mL | 1.6111 mL | 2.0139 mL |
| 100 mM | 0.0403 mL | 0.2014 mL | 0.4028 mL | 0.8055 mL | 1.0069 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 1,2,4-Benzenetriol
Catalog No.:BCC8409
CAS No.:533-73-3
- Sesamol
Catalog No.:BCN2594
CAS No.:533-31-3
- Tigloidine
Catalog No.:BCN1945
CAS No.:533-08-4
- Fmoc-Cys(Bzl)-OH
Catalog No.:BCC3475
CAS No.:53298-33-2
- CV 1808
Catalog No.:BCC7163
CAS No.:53296-10-9
- [Ala11,D-Leu15]-Orexin B
Catalog No.:BCC5877
CAS No.:532932-99-3
- Boc-Tyr(Me)-OH
Catalog No.:BCC3268
CAS No.:53267-93-9
- H-Sar-NH2.HCl
Catalog No.:BCC3333
CAS No.:5325-64-4
- 2''-O-Galloylhyperin
Catalog No.:BCN1218
CAS No.:53209-27-1
- Coixol
Catalog No.:BCN5703
CAS No.:532-91-2
- ar-Turmerone
Catalog No.:BCN7516
CAS No.:532-65-0
- Euparin
Catalog No.:BCN7191
CAS No.:532-48-9
- Benzyl carbazate
Catalog No.:BCC8872
CAS No.:5331-43-1
- Isogosferol
Catalog No.:BCN5704
CAS No.:53319-52-1
- PP 3
Catalog No.:BCC7486
CAS No.:5334-30-5
- Z-Leu-OH.DCHA
Catalog No.:BCC2765
CAS No.:53363-87-4
- Boc-N-Me-Leu-OH
Catalog No.:BCC2616
CAS No.:53363-89-6
- trans,trans-Bis(4-fluorobenzal)acetone
Catalog No.:BCC9180
CAS No.:53369-00-9
- 1,3,5-Trihydroxy-4-prenylxanthone
Catalog No.:BCN5705
CAS No.:53377-61-0
- Isovitexin 2'-O-arabinoside
Catalog No.:BCN7826
CAS No.:53382-71-1
- Erteberel (LY500307)
Catalog No.:BCC4491
CAS No.:533884-09-2
- 2-Aminodiphenylamine
Catalog No.:BCC8548
CAS No.:534-85-0
- D-Leu-ol
Catalog No.:BCC2583
CAS No.:53448-09-2
- 2-Aminonicotinic acid
Catalog No.:BCC8552
CAS No.:5345-47-1
Uremic Toxins Induce ET-1 Release by Human Proximal Tubule Cells, which Regulates Organic Cation Uptake Time-Dependently.[Pubmed:26132391]
Cells. 2015 Jun 26;4(3):234-52.
In renal failure, the systemic accumulation of uremic waste products is strongly associated with the development of a chronic inflammatory state. Here, the effect of cationic uremic toxins on the release of inflammatory cytokines and endothelin-1 (ET-1) was investigated in conditionally immortalized proximal tubule epithelial cells (ciPTEC). Additionally, we examined the effects of ET-1 on the cellular uptake mediated by organic cation transporters (OCTs). Exposure of ciPTEC to cationic uremic toxins initiated production of the inflammatory cytokines IL-6 (117 +/- 3%, p < 0.001), IL-8 (122 +/- 3%, p < 0.001), and ET-1 (134 +/- 5%, p < 0.001). This was accompanied by a down-regulation of OCT mediated 4-(4-(dimethylamino)styryl)-N-methylpyridinium-iodide (ASP+) uptake in ciPTEC at 30 min (23 +/- 4%, p < 0.001), which restored within 60 min of incubation. Exposure to ET-1 for 24 h increased the ASP+ uptake significantly (20 +/- 5%, p < 0.001). These effects could be blocked by BQ-788, indicating activation of an ET-B-receptor-mediated signaling pathway. Downstream the receptor, iNOS inhibition by (N(G)-monomethyl-l-arginine) L-NMMA acetate or aminoguanidine, as well as protein kinase C activation, ameliorated the short-term effects. These results indicate that uremia results in the release of cytokines and ET-1 from human proximal tubule cells, in vitro. Furthermore, ET-1 exposure was found to regulate proximal tubular OCT transport activity in a differential, time-dependent, fashion.
N-nitro-L-arginine and N-monomethyl-L-arginine exhibit a different pattern of inactivation toward the three nitric oxide synthases.[Pubmed:7540822]
Arch Biochem Biophys. 1995 Jun 20;320(1):170-6.
The ability of NG-nitro-L-arginine (NNA) and NG-methyl-L-arginine (NMMA) to inactivate native neuronal, endothelial cell, and macrophage nitric oxide synthases (nNOS, eNOS, and iNOS, respectively) was investigated. Each NOS isozyme (plus cofactors) was preincubated with either NNA or NMMA and then assayed for remaining activity by measuring the conversion of labeled L-arginine to labeled L-citrulline. Consistent with previous reports (Olken, N. M., et al., Biochem. Biophys. Res. Commun. 177, 828-833, 1991), NMMA was a mechanism-based irreversible inhibitor of iNOS, exhibiting time- and concentration-dependent inactivation of iNOS with a KI equal to 2.6 microM and a kinact equal to 0.042 min-1. When assayed without a preincubation period, NMMA exhibited typical reversible inhibition of iNOS (Ki = 3.9 microM). NMMA also reversibly inhibited nNOS and the eNOS with Ki equal to 0.65 and 0.7 microM, respectively. However, NMMA did not inactivate eNOS at concentrations up to 10 microM. In the presence, but not the absence, of 4 microM tetrahydrobiopterin, NMMA inactivated nNOS with a kinact equal to 0.022 min-1 and a KI equal to 2.0 microM. Since NNA did not inactivate iNOS at concentrations up to 25 microM, NNA is strictly a reversible inhibitor of iNOS (Ki = 8.1 microM). Neuronal NOS and eNOS, however, were rapidly inactivated by NNA with kintact equal to 0.083 and 0.047 min-1 and KI equal to 0.09 and 0.02 microM, respectively, when preincubated with NNA. Tetrahydrobiopterin did not affect the rate of inactivation of nNOS by NNA. In all cases, L-arginine protected against inactivation, suggesting that inactivation occurs at or near the active site. Thus, inactivation of the three NOS isozymes with NMMA and NNA reveals active-site differences between the isoforms.
Inactivation of macrophage nitric oxide synthase activity by NG-methyl-L-arginine.[Pubmed:2049105]
Biochem Biophys Res Commun. 1991 Jun 14;177(2):828-33.
.N = O synthase catalyzes the oxidation of one of the two chemically equivalent guanido nitrogens of L-arginine to nitric oxide (.N = O). NG-Methyl-L-arginine has been previously characterized as a potent competitive inhibitor of both major types of .N = O synthases. Initial rate kinetics were performed with a spectrophotometric assay based on the oxidation of oxy- to methemoglobin by .N = O. NG-Methyl-L-arginine was a competitive inhibitor of .N = O synthase activity derived from activated murine macrophages with a Ki of 6.2 microM. When the enzyme was pre-incubated in the presence of the required cofactors NADPH and tetrahydrobiopterin, time- and concentration-dependent irreversible inactivation of the activity was observed. At 37 degrees C the kinact was 0.050 min-1. This inactivation process exhibited substrate protection, saturation kinetics and required the cofactors necessary for enzymatic turnover. These data indicate that NG-methyl-L-arginine acts as a mechanism-based enzyme inactivator of murine macrophage .N = O synthase.
Characterization of three inhibitors of endothelial nitric oxide synthase in vitro and in vivo.[Pubmed:1706208]
Br J Pharmacol. 1990 Nov;101(3):746-52.
1. Three analogues of L-arginine were characterized as inhibitors of endothelial nitric oxide (NO) synthase by measuring their effect on the endothelial NO synthase from porcine aortae, on the vascular tone of rings of rat aorta and on the blood pressure of the anaesthetized rat. 2. NG-monomethyl-L-arginine (L-NMMA), N-iminoethyl-L-ornithine (L-NIO) and NG-nitro-L-arginine methyl ester (L-NAME; all at 0.1-100 microM) caused concentration-dependent inhibition of the Ca2(+)-dependent endothelial NO synthase from porcine aortae. 3. L-NMMA, L-NIO and L-NAME caused an endothelium-dependent contraction and an inhibition of the endothelium-dependent relaxation induced by acetylcholine (ACh) in aortic rings. 4. L-NMMA, L-NIO and L-NAME (0.03-300 mg kg-1, i.v.) induced a dose-dependent increase in mean systemic arterial blood pressure accompanied by bradycardia. 5. L-NMMA, L-NIO and L-NAME (100 mg kg-1, i.v.) inhibited significantly the hypotensive responses to ACh and bradykinin. 6. The increase in blood pressure and bradycardia produced by these compounds were reversed by L-arginine (30-100 mg kg-1, i.v.) in a dose-dependent manner. 7. All of these effects were enantiomer specific. 8. These results indicate that L-NMMA, L-NIO and L-NAME are inhibitors of NO synthase in the vascular endothelium and confirm the important role of NO synthesis in the maintenance of vascular tone and blood pressure.
Identification of arginine as a precursor of endothelium-derived relaxing factor.[Pubmed:3263652]
Proc Natl Acad Sci U S A. 1988 Nov;85(22):8664-7.
Nitric oxide (NO) is a major endothelium-derived relaxing factor (EDRF) released in response to vasodilating amines, peptides, proteins, ionophores, and nucleotides. EDRF is an important regulator of smooth muscle tone and platelet aggregation and adhesion. Histamine and acetylcholine relax the intact norepinephrine-constricted guinea pig pulmonary artery by an EDRF-dependent mechanism in a medium free of amino acids. N omega-Monomethylarginine (N-MeArg; 0.25 mM) inhibited this relaxation by 64-73%. Inhibition by N-MeArg developed rapidly and was immediately and completely reversed by excess L-arginine but not by D-arginine or by citrulline. N-MeArg did not diminish relaxation induced by nitroprusside, an NO-generating agent, indicating that N-MeArg acts on endothelium rather than on smooth muscle. These observations strongly suggest that, in the intact guinea pig pulmonary artery, EDRF originates from enzymatic action on the guanido nitrogen(s) of an endogenous pool of arginine. This is strikingly similar to the origin of reactive nitrogen intermediates in activated macrophages.


