EuparinCAS# 532-48-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 532-48-9 | SDF | Download SDF |
| PubChem ID | 119039 | Appearance | Powder |
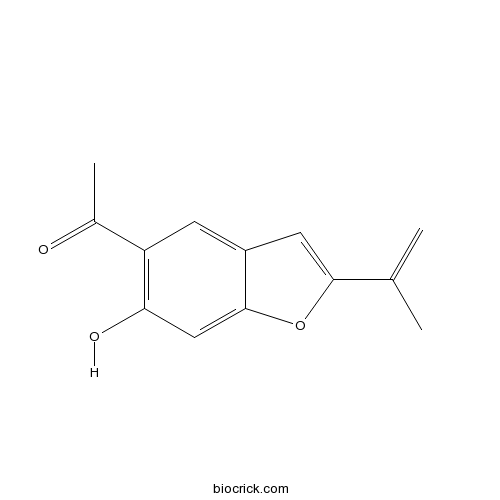
| Formula | C13H12O3 | M.Wt | 216.23 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 1-(6-hydroxy-2-prop-1-en-2-yl-1-benzofuran-5-yl)ethanone | ||
| SMILES | CC(=C)C1=CC2=CC(=C(C=C2O1)O)C(=O)C | ||
| Standard InChIKey | OPUFDNZTKHPZHM-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C13H12O3/c1-7(2)12-5-9-4-10(8(3)14)11(15)6-13(9)16-12/h4-6,15H,1H2,2-3H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Euparin has antiviral activity, it exerts its effect during the early events of the replication cycle, from the virus adsorption to cells up to the first twenty minutes after infection. 2. Euparin exhibits a moderate antioxidant activity. 3. Euparin exhibits antifungal activity against Trichophyton mentagrophytes. |
| Targets | Antifection |

Euparin Dilution Calculator

Euparin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.6247 mL | 23.1235 mL | 46.2471 mL | 92.4941 mL | 115.6176 mL |
| 5 mM | 0.9249 mL | 4.6247 mL | 9.2494 mL | 18.4988 mL | 23.1235 mL |
| 10 mM | 0.4625 mL | 2.3124 mL | 4.6247 mL | 9.2494 mL | 11.5618 mL |
| 50 mM | 0.0925 mL | 0.4625 mL | 0.9249 mL | 1.8499 mL | 2.3124 mL |
| 100 mM | 0.0462 mL | 0.2312 mL | 0.4625 mL | 0.9249 mL | 1.1562 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Tropinone
Catalog No.:BCN1935
CAS No.:532-24-1
- Anethole trithione
Catalog No.:BCN8510
CAS No.:532-11-6
- Methyocarbamol
Catalog No.:BCC3813
CAS No.:532-03-6
- Etomidate hydrochloride
Catalog No.:BCC4255
CAS No.:53188-20-8
- Fagomine
Catalog No.:BCC1569
CAS No.:53185-12-9
- 6-Aminoindole
Catalog No.:BCC8763
CAS No.:5318-27-4
- Pirfenidone
Catalog No.:BCC5086
CAS No.:53179-13-8
- Acemetacin
Catalog No.:BCC4424
CAS No.:53164-05-9
- Delphinidin-3-sambubioside chloride
Catalog No.:BCN3148
CAS No.:53158-73-9
- Euscaphic acid
Catalog No.:BCN5702
CAS No.:53155-25-2
- Buprenorphine hydrochloride
Catalog No.:BCC5215
CAS No.:53152-21-9
- Rapamycin (Sirolimus)
Catalog No.:BCC3592
CAS No.:53123-88-9
- ar-Turmerone
Catalog No.:BCN7516
CAS No.:532-65-0
- Coixol
Catalog No.:BCN5703
CAS No.:532-91-2
- 2''-O-Galloylhyperin
Catalog No.:BCN1218
CAS No.:53209-27-1
- H-Sar-NH2.HCl
Catalog No.:BCC3333
CAS No.:5325-64-4
- Boc-Tyr(Me)-OH
Catalog No.:BCC3268
CAS No.:53267-93-9
- [Ala11,D-Leu15]-Orexin B
Catalog No.:BCC5877
CAS No.:532932-99-3
- CV 1808
Catalog No.:BCC7163
CAS No.:53296-10-9
- Fmoc-Cys(Bzl)-OH
Catalog No.:BCC3475
CAS No.:53298-33-2
- Tigloidine
Catalog No.:BCN1945
CAS No.:533-08-4
- Sesamol
Catalog No.:BCN2594
CAS No.:533-31-3
- 1,2,4-Benzenetriol
Catalog No.:BCC8409
CAS No.:533-73-3
- L-NMMA acetate
Catalog No.:BCC6788
CAS No.:53308-83-1
Phytogrowth-inhibitory and antifungal constituents of Helianthella quinquenervis.[Pubmed:8882437]
J Nat Prod. 1996 Mar;59(3):323-6.
Investigation on the roots of Helianthella quinquenervis (Hook.) A. Gray (Asteraceae), led to the isolation of one new benzofuran (6-methoxy-tremetone (1)) and a new prenylacetophenone (4-beta-D-(glucopyranosyloxy)-3-[3-methoxy-trans-isopenten-1 -yl] acetophenone (3)). In addition, 6-hydroxy-3-methoxytremetone (2), encecalin (6), Euparin (5), demethylencecalin (4), and angelic acid were obtained. Structural assignments of the isolated compounds were based on spectroscopic and spectrometric analysis. Natural products 1-4 showed marginal cytotoxicity against three human tumor cell lines [MCF-7, A-549, and HT-29]. Compounds 4 and 6 inhibited the radicle growth of Amaranthus hypochondriacus and Echinochloa crusgalli. Furthermore, substances 4-6 exhibited antifungal activity against Trichophyton mentagrophytes.
In vitro evaluation of cytotoxic activity of the ethanol extract and isolated compounds from the corms of Liatris spicata (L.) willd on HepG2.[Pubmed:27712097]
Nat Prod Res. 2017 Jun;31(11):1325-1328.
Investigation of the ethanol extract of the corms of Liatris spicata (L.) willd led to the isolation of two sterols: stigmasterol and its 3-O-glucoside, a triterpene: obtusifoliyl acetate, two benzofurans: Euparin and 6-hydroxy-3-methoxytremetone, three phenolic acids: protocatechuic, vanillic and ferulic acid and a sesquiterpene lactone igalan. The structures of the isolated compounds were established on the basis of physicochemical properties and spectral analysis (IR, EI/MS, (1)H NMR and (13)C NMR). The ethanol extract and its isolated compounds evidenced cytotoxic activities against human liver cancer cell line (HepG2), where igalan showed the highest potency (3.83 +/- 0.043) mug/mL, its effect was comparable to that of the standard drug doxorubicin(R) (3.73 +/- 0.036) mug/mL.
Antipoliovirus Activity of the Organic Extract of Eupatorium buniifolium: Isolation of Euparin as an Active Compound.[Pubmed:23956770]
Evid Based Complement Alternat Med. 2013;2013:402364.
The antiviral activity of the organic extract (OE) of Eupatorium buniifolium against poliovirus type 1 was determined by in vitro assays with an effective concentration 50 (EC50) of 23.3 +/- 3.3 microg/mL. Bioassay-guided fractionation of the OE allowed the isolation of an active principle that was identified by spectroscopic methods ((1)H- and (13)C-NMR, EI-MS, UV, and IR spectroscopy) as the benzofuran Euparin. The plaque reduction assay in Vero cells was used to assess the antiviral activity of Euparin against poliovirus types 1, 2, and 3 with EC50 values of 0.47, 0.12, and 0.15 microg/mL, respectively. Moreover, this compound showed high selectivity indexes of 284.9, 1068, and 854.7, respectively. In order to identify the mechanism by which Euparin exerts its antiviral activity, the virucidal effect, the pretreatment of Vero cells, and the time of action on one viral replication cycle were evaluated. Results obtained demonstrated that Euparin exerts its effect during the early events of the replication cycle, from the virus adsorption to cells up to the first twenty minutes after infection. This is the first report on the presence of Euparin in E. buniifolium and its antiviral activity.


