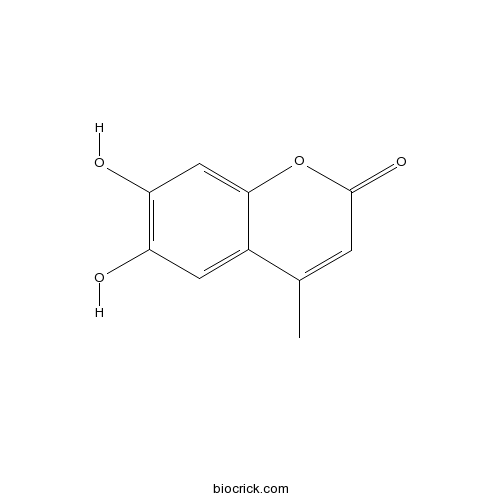
6,7-Dihydroxy-4-MethylcoumarinCAS# 529-84-0 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 529-84-0 | SDF | Download SDF |
| PubChem ID | 5319502 | Appearance | Powder |
| Formula | C10H8O4 | M.Wt | 192 |
| Type of Compound | Coumarins | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 6,7-dihydroxy-4-methylchromen-2-one | ||
| SMILES | CC1=CC(=O)OC2=CC(=C(C=C12)O)O | ||
| Standard InChIKey | KVOJTUXGYQVLAJ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C10H8O4/c1-5-2-10(13)14-9-4-8(12)7(11)3-6(5)9/h2-4,11-12H,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. 4-Methylesculetin has cytotoxicity. 2. 4-Methylesculetin displays a potent metal chelating agent. 3. 4-Methylesculetin could be an effective agent to treat arthritis and associated secondary complications like oxidative stress. 4. 4-Methylesculetin inhibits pancreatic cancer growth and metastasis by inhibition of hyaluronan synthesis. 5. 4-Methylesculetin has great anti-oxidant and anti-inflammatory activities, it has a promising potentiality to treat inflammatory diseases, especially those related to reactive oxygen species, as inflammatory bowel disease. |
| Targets | IL Receptor | TNF-α | COX | PGE | MMP(e.g.TIMP) | NF-kB | Akt | ROS |

6,7-Dihydroxy-4-Methylcoumarin Dilution Calculator

6,7-Dihydroxy-4-Methylcoumarin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.2083 mL | 26.0417 mL | 52.0833 mL | 104.1667 mL | 130.2083 mL |
| 5 mM | 1.0417 mL | 5.2083 mL | 10.4167 mL | 20.8333 mL | 26.0417 mL |
| 10 mM | 0.5208 mL | 2.6042 mL | 5.2083 mL | 10.4167 mL | 13.0208 mL |
| 50 mM | 0.1042 mL | 0.5208 mL | 1.0417 mL | 2.0833 mL | 2.6042 mL |
| 100 mM | 0.0521 mL | 0.2604 mL | 0.5208 mL | 1.0417 mL | 1.3021 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Euxanthone
Catalog No.:BCN5694
CAS No.:529-61-3
- Genistin
Catalog No.:BCN2396
CAS No.:529-59-9
- Prunin
Catalog No.:BCN5693
CAS No.:529-55-5
- Scutellarein
Catalog No.:BCN5380
CAS No.:529-53-3
- Azaleatin
Catalog No.:BCN8207
CAS No.:529-51-1
- Gentisein
Catalog No.:BCN3356
CAS No.:529-49-7
- Myricetin
Catalog No.:BCN5692
CAS No.:529-44-2
- Ombuin
Catalog No.:BCN5691
CAS No.:529-40-8
- Chamazulene
Catalog No.:BCC8145
CAS No.:529-05-5
- 20-Hydroxyecdysone
Catalog No.:BCN5688
CAS No.:5289-74-7
- Olean-12-ene-3,11-diol
Catalog No.:BCN5686
CAS No.:5282-14-4
- Cearoin
Catalog No.:BCN7772
CAS No.:52811-37-7
- Motilin (human, porcine)
Catalog No.:BCC5894
CAS No.:52906-92-0
- PRIMA-1MET
Catalog No.:BCC2414
CAS No.:5291-32-7
- Dammaradienyl acetate
Catalog No.:BCN5689
CAS No.:52914-31-5
- Neosophoramine
Catalog No.:BCN5690
CAS No.:52932-74-8
- Nanaomycin A
Catalog No.:BCC3611
CAS No.:52934-83-5
- N-(2,6-Diphenylmethyl)-1-piperazine acetylamine
Catalog No.:BCC9052
CAS No.:5294-61-1
- Genz-644282
Catalog No.:BCC1592
CAS No.:529488-28-6
- Ajugol
Catalog No.:BCN2883
CAS No.:52949-83-4
- Acetylisocupressic acid
Catalog No.:BCN5695
CAS No.:52992-82-2
- Prednisone
Catalog No.:BCC4957
CAS No.:53-03-2
- Estrone
Catalog No.:BCN2201
CAS No.:53-16-7
- Mitotane (Lsodren)
Catalog No.:BCC3815
CAS No.:53-19-0
UDP-Glucuronosyltransferases 1A6 and 1A9 are the Major Isozymes Responsible for the 7-O-Glucuronidation of Esculetin and 4-Methylesculetin in Human Liver Microsomes.[Pubmed:25854527]
Drug Metab Dispos. 2015 Jul;43(7):977-83.
Esculetin (6,7-dihydroxycoumarin, ET) and 4-methylesculetin (6,7-Dihydroxy-4-Methylcoumarin, 4-ME) are typical coumarin derivatives that are attracting considerable attention because of their wide spectrum of biologic activities, but their metabolism remains unknown. This study aimed to elucidate the in vitro UDP-glucuronosyltransferase (UGT) metabolism characteristics of ET and 4-ME. 7-O-monoglucuronide esculetin (ET-G) and 7-O-monoglucuronide 4-methylesculetin (4-ME-G) were identified by liquid chromatography-mass spectrometry (LC-MS) and (1)H-nuclear magnetic resonance ((1)HNMR) when ET or 4-ME was incubated with human liver (HLM) in the presence of UDP-glucuronic acid. Screening assays with 12 human expressed UGTs demonstrated that the formations of ET-G and 4-ME-G were almost exclusively catalyzed by UGT1A6 and UGT1A9. Phenylbutazone and carvacrol (UGT1A6 and UGT1A9 chemical inhibitors, respectively) at different concentrations (50, 100, and 200 muM) significantly inhibited the formation of glucuronidates of ET and 4-ME in HLM, UGT1A6, and UGT1A9 when the concentrations of ET and 4-ME ranged from 10 to 300 muM (P < 0.05). Clearance rates of ET in HLM, HIM, UGT1A6, and UGT1A9 were 0.54, 0.16, 0.69, and 0.14 ml/min/mg, respectively. Corresponding clearance rates values of 4-ME were 0.59, 0.03, 0.14, and 0.04 ml/min/mg, respectively. In conclusion, 7-O-monoglucuronidation by UGT1A6 and UGT1A9 was the predominant UGT metabolic pathway for both ET and 4-ME in vitro. The liver is probably the major contributor to the glucuronidation metabolism of ET and 4-ME. ET showed more rapid metabolism than 4-ME in glucuronidation.
Structure-activity relationship of dihydroxy-4-methylcoumarins as powerful antioxidants: correlation between experimental & theoretical data and synergistic effect.[Pubmed:20600568]
Biochimie. 2010 Sep;92(9):1089-100.
The chain-breaking antioxidant activities of eight coumarins [7-hydroxy-4-methylcoumarin (1), 5,7-dihydroxy-4-methylcoumarin (2), 6,7-Dihydroxy-4-Methylcoumarin (3), 6,7-dihydroxycoumarin (4), 7,8-dihydroxy-4-methylcoumarin (5), ethyl 2-(7,8-dihydroxy-4-methylcoumar-3-yl)-acetate (6), 7,8-diacetoxy-4-methylcoumarin (7) and ethyl 2-(7,8-diacetoxy-4-methylcoumar-3-yl)-acetate (8)] during bulk lipid autoxidation at 37 degrees C and 80 degrees C in concentrations of 0.01-1.0 mM and their radical scavenging activities at 25 degrees C using TLC-DPPH test have been studied and compared. It has been found that the o-dihydroxycoumarins 3-6 demonstrated excellent activity as antioxidants and radical scavengers, much better than the m-dihydroxy analogue 2 and the monohydroxycoumarin 1. The substitution at the C-3 position did not have any effect either on the chain-breaking antioxidant activity or on the radical scavenging activity of the 7,8-dihydroxy- and 7,8-diacetoxy-4-methylcoumarins 6 and 8. The comparison with DL-alpha-tocopherol (TOH), caffeic acid (CA) and p-coumaric acid (p-CumA) showed that antioxidant efficiency decreases in the following sequence: TOH>CA>3>4>6>5>2>1=7=8=p-CumA. Theoretical calculations and the "Lipinski's Rule of Five" were used for explaining the structure-activity relationships and pharmacokinetic behavior. A higher TGSO oxidation stability was observed in the presence of equimolar (1:1) binary mixtures of coumarins with TOH (1+TOH, 3+TOH and 5+TOH). However, the synergism (14%) was observed only for the binary mixture of 5 + TOH.
Structural requirements of hydroxylated coumarins for in vitro anti-Helicobacter pylori activity.[Pubmed:14598616]
In Vivo. 2003 Sep-Oct;17(5):509-12.
We have previously found that a 7-hydroxycoumarin derivative has potent anti-Helicobacter pylori (H. pylori) activity, comparable with metronidazole. In this report, we describe the structural requirement for the anti-H. pylori activity of several hydroxylated coumarins (1-23). It was found that 7-hydroxy-4-methylcoumarin (6), 6,7-Dihydroxy-4-Methylcoumarin (8), 6-hydroxy-7-methoxy-4-methylcoumarin (10) and 5,7-dihydroxycyclopentanocoumarin (21) showed comparable anti-H. pylori activity with metronidazole. The presence of 7- and/or 6-hydroxyl groups seems to be essential to display higher anti-H. pylori activity. Their activities depended on the number and position of the hydroxyl group on the benzenoid ring of the coumarin system. Methylation of the hydroxy group generally diminished the activity. In hydroxylated coumarins, the methyl group at C-4 position enhanced the activity. The inhibitory activity of coumarins (1-23) against jack bean urease was examined, but no coumarins showed any inhibition at 160 micrograms/mL.
Effect of coumarins on HL-60 cell differentiation.[Pubmed:10953319]
Anticancer Res. 2000 Jul-Aug;20(4):2505-12.
Twenty-eight coumarins, including 7 furocoumarins, were examined for their activity of induction of terminal differentiation of human promyelocytic leukemia cells (HL-60) by nitro blue tetrazolium (NBT) reducing, nonspecific esterase, specific esterase and phagocytic activities. Esculetin, nordalbergin, 6,7-Dihydroxy-4-Methylcoumarin and imperatorin had strong activity among the coumarins examined. HL-60 cells treated with these coumarins differentiated into mature monocyte/macrophage. The structure-activity relationship established from the results revealed that 6,7-dihydroxy moiety had an important role in the induction of differentiation of HL-60.
Pharmacological and biochemical actions of simple coumarins: natural products with therapeutic potential.[Pubmed:8853310]
Gen Pharmacol. 1996 Jun;27(4):713-22.
1. More than 300 coumarins have been identified from natural sources, especially green plants. The pharmacological and biochemical properties and therapeutic applications of simple coumarins depend upon the pattern of substitution. More complex related compounds based on the coumarin nucleus include the dicoumarol/warfarin anticoagulants, aflatoxins and the psoralens (photosensitizing agents). 2. Coumarin itself (1,2-benzopyrone) has long-established efficacy in slow-onset long-term reduction of lymphoedema in man, as confirmed in recent double-blind trials against elephantiasis and postmastectomy swelling of the arm. The mechanism of action is uncertain, but may involve macrophage-induced proteolysis of oedema protein. However, coumarin has low absolute bioavailability in man (< 5%), due to extensive first-pass hepatic conversion to 7-hydroxycoumarin followed by glucuronidation. It may, therefore, be a prodrug. 3. Scoparone (6,7-dimethoxycoumarin) has been purified from the hypolipidaemic Chinese herb Artemisia scoparia and shown to reduce the proliferative responses of human peripheral mononuclear cells, to relax smooth muscle, to reduce total cholesterol and triglycerides and to retard the characteristic pathomorphological changes in hypercholesterolaemic diabetic rabbits. Various properties of scoparone were suggested to account for these findings, including ability to scavenge reactive oxygen species, inhibition of tyrosine kinases and potentiation of prostaglandin generation. 4. Osthole (7-methoxy-8-[3-methylpent-2-enyl]coumarin) from Angelica pubescens, used also in Chinese medicine, causes hypotension in vivo, and inhibits platelet aggregation and smooth muscle contraction in vitro. It may interfere with calcium influx and with cyclic nucleotide phosphodiesterases. 5. Cloricromene, a synthetic coumarin derivative, also possesses antithrombotic antiplatelet actions, inhibits PMN neutrophil function and causes vasodilatation. Some of these properties of cloricromene have been ascribed to inhibition of arachidonate release from membrane phospholipids. 6. Simple coumarins possessing ortho-dihydroxy functions, such as fraxetin and 4-methyldaphnetin, are potent inhibitors (low micromolar) of lipid peroxidation and scavengers of superoxide anion radicals and of aqueous alkylperoxyl radicals, but may be pro-oxidant (enhancing generation of hydroxyl radicals) in the presence of free iron ions. These coumarins also inhibit the proinflammatory 5-lipoxygenase enzyme at micromolar concentrations. Another related coumarin, 5,7-dihydroxy-4-methylcoumarin, is of special interest as it inhibits lipid peroxidation, and scavenges alkylperoxyl and superoxide radicals. Unlike most other simple coumarins studied, 5,7-dihydroxy-4-methylcoumarin also scavenges hypochlorous acid, and is a potent inhibitor of cyclo-oxygenase, but is not pro-oxidant. 7. 5,7- and 6,7-Dihydroxy-4-Methylcoumarin both reduced the duration of ventricular fibrillation in postischaemic reperfused isolated perfused rat hearts (in which oxygen-derived free radicals are implicated), showing that these antioxidant coumarins possess beneficial properties in this pathophysiological model. 8. In view of the established low toxicity, relative cheapness, presence in the diet and occurrence in various herbal remedies of coumarins, it appears prudent to evaluate their properties and applications further.


