OmbuinCAS# 529-40-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 529-40-8 | SDF | Download SDF |
| PubChem ID | 5320287 | Appearance | Yellow powder |
| Formula | C17H14O7 | M.Wt | 330.3 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
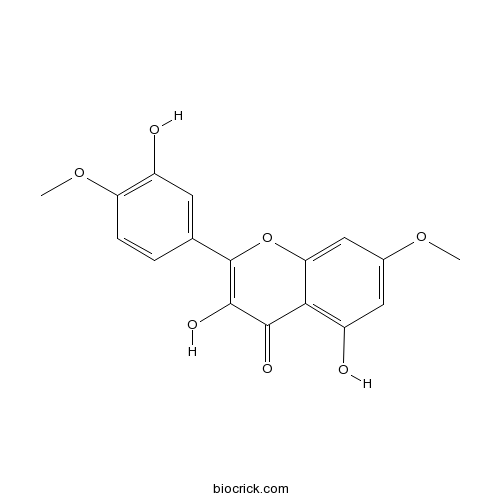
| Chemical Name | 3,5-dihydroxy-2-(3-hydroxy-4-methoxyphenyl)-7-methoxychromen-4-one | ||
| SMILES | COC1=C(C=C(C=C1)C2=C(C(=O)C3=C(C=C(C=C3O2)OC)O)O)O | ||
| Standard InChIKey | BWORNNDZQGOKBY-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C17H14O7/c1-22-9-6-11(19)14-13(7-9)24-17(16(21)15(14)20)8-3-4-12(23-2)10(18)5-8/h3-7,18-19,21H,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Ombuin has anti-inflammary activity, it inhibits some macrophage functions involved in the inflammatory process. Ombuin significantly and dose dependently inhibits lipopolysaccharide (LPS) and interferon (IFN)-gamma induced nitric oxide (NO), and cytokines [tumor necrosis factor (TNF)-alpha and interleukin (IL)-12]. |
| Targets | NO | IFN-γ | IL Receptor | TNF-α |
| In vitro | Antiinflammatory activities of flavonoids and a triterpene caffeate isolated from Bauhinia variegata.[Pubmed: 18384188]Phytother Res. 2008 Jul;22(7):957-62.In the continuing search for novel antiinflammatory agents, six flavonoids, namely kaempferol (1), Ombuin (2), kaempferol 7,4'-dimethyl ether 3-O-beta-D-glucopyranoside (3), kaempferol 3-O-beta-D-glucopyranoside (4), isorhamnetin 3-O-beta-D-glucopyranoside (5) and hesperidin (6), together with one triterpene caffeate, 3beta-trans-(3,4-dihydroxycinnamoyloxy)olean-12-en-28-oic acid (7) were isolated from the non-woody aerial parts of Bauhinia variegata. Licochalcone A: an inducer of cell differentiation and cytotoxic agent from Pogostemon cablin.[Pubmed: 9690352]Planta Med. 1998 Jun;64(5):464-6.Licochalcone A (1), Ombuin (2), and 5,7-dihydroxy-3',4'-dimethoxyflavanone (3) were isolated from the aerial parts of Pogostemon cablin by cytotoxicity-guided fractionation. |

Ombuin Dilution Calculator

Ombuin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.0276 mL | 15.1378 mL | 30.2755 mL | 60.551 mL | 75.6888 mL |
| 5 mM | 0.6055 mL | 3.0276 mL | 6.0551 mL | 12.1102 mL | 15.1378 mL |
| 10 mM | 0.3028 mL | 1.5138 mL | 3.0276 mL | 6.0551 mL | 7.5689 mL |
| 50 mM | 0.0606 mL | 0.3028 mL | 0.6055 mL | 1.211 mL | 1.5138 mL |
| 100 mM | 0.0303 mL | 0.1514 mL | 0.3028 mL | 0.6055 mL | 0.7569 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Chamazulene
Catalog No.:BCC8145
CAS No.:529-05-5
- 20-Hydroxyecdysone
Catalog No.:BCN5688
CAS No.:5289-74-7
- Olean-12-ene-3,11-diol
Catalog No.:BCN5686
CAS No.:5282-14-4
- Cearoin
Catalog No.:BCN7772
CAS No.:52811-37-7
- Dalbergiphenol
Catalog No.:BCN7451
CAS No.:52811-31-1
- L-Quisqualic acid
Catalog No.:BCC6568
CAS No.:52809-07-1
- Isobavachromene
Catalog No.:BCN3192
CAS No.:52801-22-6
- (±)-Galgravin
Catalog No.:BCN8283
CAS No.:528-63-2
- Cyanidin Chloride
Catalog No.:BCN1231
CAS No.:528-58-5
- Delphinidin chloride
Catalog No.:BCN3015
CAS No.:528-53-0
- Fisetin
Catalog No.:BCN5024
CAS No.:528-48-3
- Magnolol
Catalog No.:BCN5687
CAS No.:528-43-8
- Myricetin
Catalog No.:BCN5692
CAS No.:529-44-2
- Gentisein
Catalog No.:BCN3356
CAS No.:529-49-7
- Azaleatin
Catalog No.:BCN8207
CAS No.:529-51-1
- Scutellarein
Catalog No.:BCN5380
CAS No.:529-53-3
- Prunin
Catalog No.:BCN5693
CAS No.:529-55-5
- Genistin
Catalog No.:BCN2396
CAS No.:529-59-9
- Euxanthone
Catalog No.:BCN5694
CAS No.:529-61-3
- 6,7-Dihydroxy-4-Methylcoumarin
Catalog No.:BCC8287
CAS No.:529-84-0
- Motilin (human, porcine)
Catalog No.:BCC5894
CAS No.:52906-92-0
- PRIMA-1MET
Catalog No.:BCC2414
CAS No.:5291-32-7
- Dammaradienyl acetate
Catalog No.:BCN5689
CAS No.:52914-31-5
- Neosophoramine
Catalog No.:BCN5690
CAS No.:52932-74-8
Antiinflammatory activities of flavonoids and a triterpene caffeate isolated from Bauhinia variegata.[Pubmed:18384188]
Phytother Res. 2008 Jul;22(7):957-62.
In the continuing search for novel antiinflammatory agents, six flavonoids, namely kaempferol (1), Ombuin (2), kaempferol 7,4'-dimethyl ether 3-O-beta-D-glucopyranoside (3), kaempferol 3-O-beta-D-glucopyranoside (4), isorhamnetin 3-O-beta-D-glucopyranoside (5) and hesperidin (6), together with one triterpene caffeate, 3beta-trans-(3,4-dihydroxycinnamoyloxy)olean-12-en-28-oic acid (7) were isolated from the non-woody aerial parts of Bauhinia variegata. Compounds 1-7 were evaluated as inhibitors of some macrophage functions involved in the inflammatory process. These seven compounds significantly and dose dependently inhibited lipopolysaccharide (LPS) and interferon (IFN)-gamma induced nitric oxide (NO), and cytokines [tumor necrosis factor (TNF)-alpha and interleukin (IL)-12]. The concentration causing a 50% inhibition (IC50) of NO, TNF-alpha and IL-12 production by compounds 1, 2 and 7 was approximately 30, 50 and 10 microM, respectively, while at 50, 200 and 40 microM compounds 3, 4, and 5, 6 showed 15-30% inhibition, respectively. On the other hand, compounds 3 and 7 showed no inhibitory effect, while compounds 1, 4-6 reduced by around 10-30% the synthesis of NO by macrophages, when inducible NO synthase was already expressed with LPS/IFN-gamma for 24 h. These experimental findings lend pharmacological support to the suggested folkloric uses of the plant B. variegata in the management of inflammatory conditions.
Licochalcone A: an inducer of cell differentiation and cytotoxic agent from Pogostemon cablin.[Pubmed:9690352]
Planta Med. 1998 Jun;64(5):464-6.
Licochalcone A (1), Ombuin (2), and 5,7-dihydroxy-3',4'-dimethoxyflavanone (3) were isolated from the aerial parts of Pogostemon cablin by cytotoxicity-guided fractionation. Compound 1 showed in vitro cytotoxicity and Pl-PLC gamma 1 inhibition activity. Treatment of promyelocytic leukemia cells (HL-60) with compound 1 induced terminal differentiation with the generation of monocyte using nonspecific acid esterase assay.


