GentiseinCAS# 529-49-7 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 529-49-7 | SDF | Download SDF |
| PubChem ID | 5281635 | Appearance | Yellow powder |
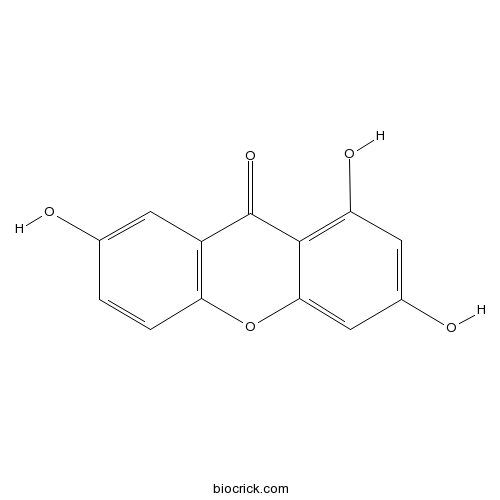
| Formula | C13H8O5 | M.Wt | 244.2 |
| Type of Compound | Xanthones | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 1,3,7-trihydroxyxanthen-9-one | ||
| SMILES | C1=CC2=C(C=C1O)C(=O)C3=C(C=C(C=C3O2)O)O | ||
| Standard InChIKey | JJUNZBRHHGLJQW-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C13H8O5/c14-6-1-2-10-8(3-6)13(17)12-9(16)4-7(15)5-11(12)18-10/h1-5,14-16H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Standard reference |
| In vitro | Effect of benzophenones from Hypericum annulatum on carbon tetrachloride-induced toxicity in freshly isolated rat hepatocytes.[Pubmed: 16571270]Redox Rep. 2006;11(1):3-8.Five benzophenones and a xanthone, isolated from Hypericum annulatum Moris, were investigated for their protective effect against carbon tetrachloride toxicity in isolated rat hepatocytes.
|
| Structure Identification | Phytochem Anal. 2007 Jan-Feb;18(1):1-6.Simultaneous determination of benzophenones and gentisein in Hypericum annulatum Moris by high-performance liquid chromatography.[Pubmed: 17260692]The content of the benzophenones, hypericophenonoside, neoannulatophenonoside, annulatophenonoside, annulatophenone, acetylannulatophenonoside and the xanthone derivative Gentisein have been determined in aerial parts, leaves, flowers and stems of Hypericum annulatum Moris.
Nat Prod Commun. 2009 Jun;4(6):803-8.Globulixanthone F, a new polyoxygenated xanthone with an isoprenoid group and two antimicrobial biflavonoids from the stem bark of Symphonia globulifera.[Pubmed: 19634326]
|

Gentisein Dilution Calculator

Gentisein Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.095 mL | 20.475 mL | 40.95 mL | 81.9001 mL | 102.3751 mL |
| 5 mM | 0.819 mL | 4.095 mL | 8.19 mL | 16.38 mL | 20.475 mL |
| 10 mM | 0.4095 mL | 2.0475 mL | 4.095 mL | 8.19 mL | 10.2375 mL |
| 50 mM | 0.0819 mL | 0.4095 mL | 0.819 mL | 1.638 mL | 2.0475 mL |
| 100 mM | 0.041 mL | 0.2048 mL | 0.4095 mL | 0.819 mL | 1.0238 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Myricetin
Catalog No.:BCN5692
CAS No.:529-44-2
- Ombuin
Catalog No.:BCN5691
CAS No.:529-40-8
- Chamazulene
Catalog No.:BCC8145
CAS No.:529-05-5
- 20-Hydroxyecdysone
Catalog No.:BCN5688
CAS No.:5289-74-7
- Olean-12-ene-3,11-diol
Catalog No.:BCN5686
CAS No.:5282-14-4
- Cearoin
Catalog No.:BCN7772
CAS No.:52811-37-7
- Dalbergiphenol
Catalog No.:BCN7451
CAS No.:52811-31-1
- L-Quisqualic acid
Catalog No.:BCC6568
CAS No.:52809-07-1
- Isobavachromene
Catalog No.:BCN3192
CAS No.:52801-22-6
- (±)-Galgravin
Catalog No.:BCN8283
CAS No.:528-63-2
- Cyanidin Chloride
Catalog No.:BCN1231
CAS No.:528-58-5
- Delphinidin chloride
Catalog No.:BCN3015
CAS No.:528-53-0
- Azaleatin
Catalog No.:BCN8207
CAS No.:529-51-1
- Scutellarein
Catalog No.:BCN5380
CAS No.:529-53-3
- Prunin
Catalog No.:BCN5693
CAS No.:529-55-5
- Genistin
Catalog No.:BCN2396
CAS No.:529-59-9
- Euxanthone
Catalog No.:BCN5694
CAS No.:529-61-3
- 6,7-Dihydroxy-4-Methylcoumarin
Catalog No.:BCC8287
CAS No.:529-84-0
- Motilin (human, porcine)
Catalog No.:BCC5894
CAS No.:52906-92-0
- PRIMA-1MET
Catalog No.:BCC2414
CAS No.:5291-32-7
- Dammaradienyl acetate
Catalog No.:BCN5689
CAS No.:52914-31-5
- Neosophoramine
Catalog No.:BCN5690
CAS No.:52932-74-8
- Nanaomycin A
Catalog No.:BCC3611
CAS No.:52934-83-5
- N-(2,6-Diphenylmethyl)-1-piperazine acetylamine
Catalog No.:BCC9052
CAS No.:5294-61-1
Effect of benzophenones from Hypericum annulatum on carbon tetrachloride-induced toxicity in freshly isolated rat hepatocytes.[Pubmed:16571270]
Redox Rep. 2006;11(1):3-8.
Five benzophenones and a xanthone, isolated from Hypericum annulatum Moris, were investigated for their protective effect against carbon tetrachloride toxicity in isolated rat hepatocytes. The benzophenones and the xanthone Gentisein were administered alone (100 microM) and in combination with CCl4 (86 microM). CCl4 undergoes dehalogenation in the liver endoplasmic reticulum. This process leads to trichlormethyl radical (*CCl3) formation, initiation of lipid peroxidation, and measurable toxic effects on the hepatocytes. The levels of thiobarbituric acid reactive substances (TBARS) were assayed as an index of lipid peroxidation (LPO). Lactate dehydrogenase (LDH) leakage, cell viability and reduced glutathione (GSH) depletion were used as signs of cytotoxicity. CCl4 significantly decreased hepatocyte viability, GSH level and increased TBARS level and LDH leakage as compared to the control. Our data indicate that 2,3',5',6-tetrahydroxy-4-methoxybenzophenone, 2-O-alpha-L-arabinofuranosyl-3',5',6-trihydroxy-4-methoxybenzophenone and 2-O-alpha-L-3'-acetylarabinofuranosyl-3',5',6-trihydroxy-4-methoxybenzophenone showed weaker toxic effects compared to CCl4 and in combination showed statistically significant protection against the toxic agent.
Simultaneous determination of benzophenones and gentisein in Hypericum annulatum Moris by high-performance liquid chromatography.[Pubmed:17260692]
Phytochem Anal. 2007 Jan-Feb;18(1):1-6.
The content of the benzophenones, hypericophenonoside, neoannulatophenonoside, annulatophenonoside, annulatophenone, acetylannulatophenonoside and the xanthone derivative Gentisein have been determined in aerial parts, leaves, flowers and stems of Hypericum annulatum Moris. Extraction of samples with methanol by magnetic stirring at room temperature allowed a good recovery of analytes (from 90.70% for Gentisein to 103.81% for annulatophenonoside) and the precision of the entire procedure was < 6.05%. The subsequent HPLC separation and quantification was achieved using a Hypersil ODS C18 column and UV detection at 290 nm. The mobile phase comprised methanol and 20 mm potassium dihydrogen phosphate (adjusted to a pH of 3.19 with o-phosphoric acid), and gradient elution mode was applied. The detection limits were 0.03, 0.02 and 0.001 microg/mL for hypericophenonoside, acetylannulatophenonoside and Gentisein, respectively. The total amounts of the phenolic compounds assayed ranged from 10.92 mg/g in stems to 82.86 mg/g in leaves. Hypericophenonoside was the dominant benzophenone present in the majority of the plant samples, being present in amounts between 7.54 +/- 0.25 mg/g in stems and 64.22 +/- 2.44 mg/g in leaves. Hypericophenonoside accounted for up to 77.50% of the components found in the leaves, whereas annulatophenonoside (6.29 +/- 0.15 mg/g) and acetylannulatophenonoside (8.95 +/- 0.09 mg/g) were detected in much lower quantities. In contrast to leaves, flowers showed a tendency towards higher contents of Gentisein (9.35 +/- 0.07 mg/g) and neoannulatophenonoside (4.72 +/- 0.04 mg/g) than the other parts assayed.
Globulixanthone F, a new polyoxygenated xanthone with an isoprenoid group and two antimicrobial biflavonoids from the stem bark of Symphonia globulifera.[Pubmed:19634326]
Nat Prod Commun. 2009 Jun;4(6):803-8.
Bioassay-guided fractionation of the stem bark of Symphonia globulifera has yielded three known xanthones, ugaxanthone (1), mbarraxanthone (2) and Gentisein (3), two biflavonoid derivatives named GB2 (4) and manniflavanone GB3 (5), and one new polyoxygenated xanthone with an isoprenoid group, named globulixanthone F (6). The structures of these compounds were elucidated by means of spectroscopic methods. The spectral data of 1 and 2 are reported here for the first time, as well as the antimicrobial activity of globulixanthone F against a range of microorganisms. We also report the total synthesis of the xanthone skeleton.


