Daturametelin ICAS# 904667-65-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 904667-65-8 | SDF | Download SDF |
| PubChem ID | 91895479 | Appearance | Powder |
| Formula | C34H48O10 | M.Wt | 616.8 |
| Type of Compound | Steroids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
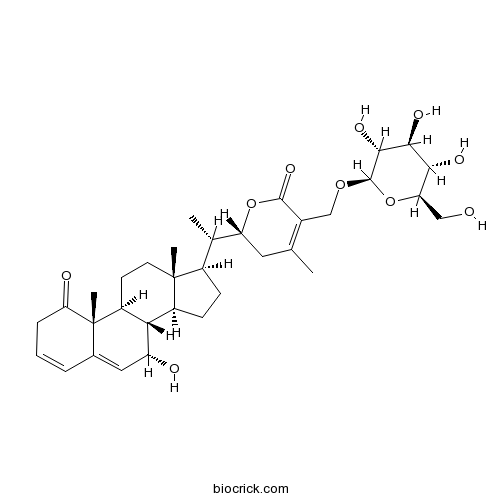
| Chemical Name | (2R)-2-[(1S)-1-[(7S,8S,9S,10R,13R,14S,17R)-7-hydroxy-10,13-dimethyl-1-oxo-2,7,8,9,11,12,14,15,16,17-decahydrocyclopenta[a]phenanthren-17-yl]ethyl]-4-methyl-5-[[(2R,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxymethyl]-2,3-dihydropyran-6-one | ||
| SMILES | CC1=C(C(=O)OC(C1)C(C)C2CCC3C2(CCC4C3C(C=C5C4(C(=O)CC=C5)C)O)C)COC6C(C(C(C(O6)CO)O)O)O | ||
| Standard InChIKey | ZKPXDCPRHDFPTL-UWOSJZGMSA-N | ||
| Standard InChI | InChI=1S/C34H48O10/c1-16-12-24(43-31(41)19(16)15-42-32-30(40)29(39)28(38)25(14-35)44-32)17(2)20-8-9-21-27-22(10-11-33(20,21)3)34(4)18(13-23(27)36)6-5-7-26(34)37/h5-6,13,17,20-25,27-30,32,35-36,38-40H,7-12,14-15H2,1-4H3/t17-,20+,21-,22-,23+,24+,25+,27-,28+,29-,30+,32+,33+,34-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| In vitro | Daturametelins H, I, and J: Three New Withanolide Glycosides from Datura metel L.[Reference: WebLink]Chemistry & Biodiversity, 2006, 3(2):180-6
|
| Animal Research | Metabolites Identification of Bioactive Compounds Daturataturin A, Daturametelin I, N-Trans-Feruloyltyramine, and Cannabisin F From the Seeds of Datura metel in Rats.[Reference: WebLink]Frontiers in Pharmacology, 2018, 9:731.Datura metel L. is a widely used traditional herbal medicine, and withanolides and amides are the two groups of main bioactive constituents in Datura metel seeds. This study aimed to elucidate the metabolism of four representative bioactive compositions containing daturataturin A (1), Daturametelin I (2), N-trans-feruloyltyramine (3), and cannabisin F (4) in rats.
|

Daturametelin I Dilution Calculator

Daturametelin I Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.6213 mL | 8.1064 mL | 16.2127 mL | 32.4254 mL | 40.5318 mL |
| 5 mM | 0.3243 mL | 1.6213 mL | 3.2425 mL | 6.4851 mL | 8.1064 mL |
| 10 mM | 0.1621 mL | 0.8106 mL | 1.6213 mL | 3.2425 mL | 4.0532 mL |
| 50 mM | 0.0324 mL | 0.1621 mL | 0.3243 mL | 0.6485 mL | 0.8106 mL |
| 100 mM | 0.0162 mL | 0.0811 mL | 0.1621 mL | 0.3243 mL | 0.4053 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Daturataturin A aglycone
Catalog No.:BCN4445
CAS No.:904665-71-0
- NU 1025
Catalog No.:BCC2454
CAS No.:90417-38-2
- Neochamaejasmine A
Catalog No.:BCN3129
CAS No.:90411-13-5
- Neochamaejasmine B
Catalog No.:BCN3130
CAS No.:90411-12-4
- Bleomycin Sulfate
Catalog No.:BCC3694
CAS No.:9041-93-4
- (-)-Indolactam V
Catalog No.:BCC7735
CAS No.:90365-57-4
- Ligularizine
Catalog No.:BCN2091
CAS No.:90364-92-4
- Neoligularidine
Catalog No.:BCN2137
CAS No.:90364-91-3
- Ligularinine
Catalog No.:BCN2117
CAS No.:90364-90-2
- Pitolisant hydrochloride
Catalog No.:BCC1863
CAS No.:903576-44-3
- Bicalutamide
Catalog No.:BCC2481
CAS No.:90357-06-5
- 7-Xylosyltaxol B
Catalog No.:BCN7675
CAS No.:90352-19-5
- Maoyerabdosin
Catalog No.:BCN3944
CAS No.:90468-72-7
- Valeriotetrate C
Catalog No.:BCN6753
CAS No.:904891-20-9
- Cryptochlorogenic acid
Catalog No.:BCN5907
CAS No.:905-99-7
- TMCB
Catalog No.:BCC7745
CAS No.:905105-89-7
- GDC-0879
Catalog No.:BCC2482
CAS No.:905281-76-7
- 2,5-dihydroxy-3-methoxy-Acetophenone
Catalog No.:BCN3780
CAS No.:90536-47-3
- Ethyl 2,4,6-trihydroxybenzoate
Catalog No.:BCN3997
CAS No.:90536-74-6
- MLN4924
Catalog No.:BCC4057
CAS No.:905579-51-3
- AZ 960
Catalog No.:BCC2197
CAS No.:905586-69-8
- Sorghumol
Catalog No.:BCN4447
CAS No.:90582-44-8
- Sorghumol acetate
Catalog No.:BCN4448
CAS No.:90582-47-1
- Tivantinib (ARQ 197)
Catalog No.:BCC3688
CAS No.:905854-02-6
Metabolites Identification of Bioactive Compounds Daturataturin A, Daturametelin I, N-Trans-Feruloyltyramine, and Cannabisin F From the Seeds of Datura metel in Rats.[Pubmed:30050436]
Front Pharmacol. 2018 Jul 9;9:731.
Datura metel L. is a widely used traditional herbal medicine, and withanolides and amides are the two groups of main bioactive constituents in Datura metel seeds. This study aimed to elucidate the metabolism of four representative bioactive compositions containing daturataturin A (1), Daturametelin I (2), N-trans-feruloyltyramine (3), and cannabisin F (4) in rats. After separately oral administration of 20 mg/kg withanolides (1, 2) and amides (3, 4) to rats, a total of 12, 24, and 21 metabolites were detected in the plasma, urine, and fecal samples, respectively. Among them, three hydroxylated metabolites, 1-M3, 2-M2, and 3-M5, were detected in plasma and rat liver microsome incubation system in high abundance. Two metabolites of 1 and 2 were unambiguously identified by comparing with reference standards. Particularly, the methylated metabolite 27alpha-methoxy-(22R)-22,26-epoxy-27-[(beta-D-glucopyranosyl)oxy]ergosta-2,4,6,24 -tetraene-1,26-dione (daturametelin L) is a new compound. The withanolides could readily get hydroxylation or methylation metabolism. Meanwhile, the phase II metabolism (glucuronidation or sulfation) was the major reaction for the amides. This is the first study on in vivo metabolism of these active compounds in seeds of Datura metel.


