NU 1025PARP inhibitor,potent and novel CAS# 90417-38-2 |
- MK-4827
Catalog No.:BCC1761
CAS No.:1038915-60-4
- BMN-673 8R,9S
Catalog No.:BCC1422
CAS No.:1207456-00-5
- XAV-939
Catalog No.:BCC1120
CAS No.:284028-89-3
- PJ34
Catalog No.:BCC1865
CAS No.:344458-19-1
- ABT-888 (Veliparib)
Catalog No.:BCC1267
CAS No.:912444-00-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 90417-38-2 | SDF | Download SDF |
| PubChem ID | 63306 | Appearance | Powder |
| Formula | C9H8N2O2 | M.Wt | 176.17 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in 1eq. NaOH and to 100 mM in DMSO | ||
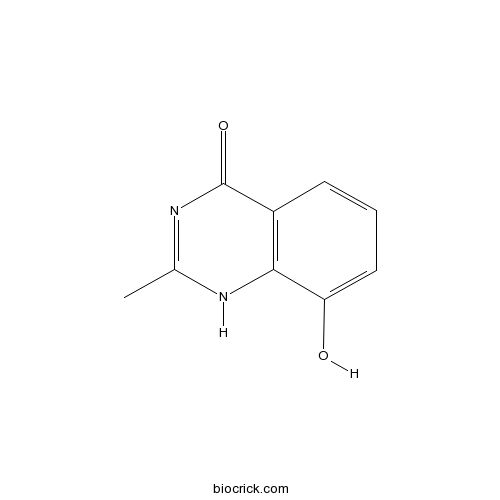
| Chemical Name | 8-hydroxy-2-methyl-1H-quinazolin-4-one | ||
| SMILES | CC1=NC(=O)C2=C(N1)C(=CC=C2)O | ||
| Standard InChIKey | YJDAOHJWLUNFLX-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C9H8N2O2/c1-5-10-8-6(9(13)11-5)3-2-4-7(8)12/h2-4,12H,1H3,(H,10,11,13) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Novel, potent inhibitor of poly(ADP-ribose) polymerase (PARP). Ki and IC50 values are 48 and 400 nM respectively. |

NU 1025 Dilution Calculator

NU 1025 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.6763 mL | 28.3817 mL | 56.7634 mL | 113.5267 mL | 141.9084 mL |
| 5 mM | 1.1353 mL | 5.6763 mL | 11.3527 mL | 22.7053 mL | 28.3817 mL |
| 10 mM | 0.5676 mL | 2.8382 mL | 5.6763 mL | 11.3527 mL | 14.1908 mL |
| 50 mM | 0.1135 mL | 0.5676 mL | 1.1353 mL | 2.2705 mL | 2.8382 mL |
| 100 mM | 0.0568 mL | 0.2838 mL | 0.5676 mL | 1.1353 mL | 1.4191 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Novel, potent inhibitor of poly(ADP-ribose) polymerase (PARP). Ki and IC50 values are 48 and 400 nM respectively.
- Neochamaejasmine A
Catalog No.:BCN3129
CAS No.:90411-13-5
- Neochamaejasmine B
Catalog No.:BCN3130
CAS No.:90411-12-4
- Bleomycin Sulfate
Catalog No.:BCC3694
CAS No.:9041-93-4
- (-)-Indolactam V
Catalog No.:BCC7735
CAS No.:90365-57-4
- Ligularizine
Catalog No.:BCN2091
CAS No.:90364-92-4
- Neoligularidine
Catalog No.:BCN2137
CAS No.:90364-91-3
- Ligularinine
Catalog No.:BCN2117
CAS No.:90364-90-2
- Pitolisant hydrochloride
Catalog No.:BCC1863
CAS No.:903576-44-3
- Bicalutamide
Catalog No.:BCC2481
CAS No.:90357-06-5
- 7-Xylosyltaxol B
Catalog No.:BCN7675
CAS No.:90352-19-5
- 10-O-Coumaroyl-10-O-deacetylasperuloside
Catalog No.:BCN7614
CAS No.:903519-82-4
- Seneciphyllinine
Catalog No.:BCN2132
CAS No.:90341-45-0
- Daturataturin A aglycone
Catalog No.:BCN4445
CAS No.:904665-71-0
- Daturametelin I
Catalog No.:BCN4446
CAS No.:904667-65-8
- Maoyerabdosin
Catalog No.:BCN3944
CAS No.:90468-72-7
- Valeriotetrate C
Catalog No.:BCN6753
CAS No.:904891-20-9
- Cryptochlorogenic acid
Catalog No.:BCN5907
CAS No.:905-99-7
- TMCB
Catalog No.:BCC7745
CAS No.:905105-89-7
- GDC-0879
Catalog No.:BCC2482
CAS No.:905281-76-7
- 2,5-dihydroxy-3-methoxy-Acetophenone
Catalog No.:BCN3780
CAS No.:90536-47-3
- Ethyl 2,4,6-trihydroxybenzoate
Catalog No.:BCN3997
CAS No.:90536-74-6
- MLN4924
Catalog No.:BCC4057
CAS No.:905579-51-3
- AZ 960
Catalog No.:BCC2197
CAS No.:905586-69-8
- Sorghumol
Catalog No.:BCN4447
CAS No.:90582-44-8
Rapamycin-resistant poly (ADP-ribose) polymerase-1 overexpression is a potential therapeutic target in lymphangioleiomyomatosis.[Pubmed:24874429]
Am J Respir Cell Mol Biol. 2014 Dec;51(6):738-49.
Lymphangioleiomyomatosis (LAM) is a female-predominant cystic lung disease that can lead to respiratory failure. LAM cells typically have inactivating tuberous sclerosis complex 2 (TSC2) mutations and mammalian target of rapamycin (mTOR) complex (mTORC) 1 activation. Clinical response to the mTORC1 inhibitors has been limited, prompting a search for additional therapy for LAM. In this study, we investigated the impact of TSC2 on the expression of poly (ADP-ribose) polymerase (PARP)-1 that initiates the DNA repair pathway, and tested the efficacy of PARP1 inhibitors in the survival of TSC2-deficient (TSC2(-)) cells. We analyzed publicly available expression arrays of TSC2(-) cells and validated the findings using real-time RT-PCR, immunoblotting, and immunohistochemistry. We examined the impact of rapamycin and Torin 1 on PARP1 expression. We also tested the effect of PARP1 inhibitors, 8-hydroxy-2-methylquinazoline-4-one and 3,4-dihydro-5[4-(1-piperindinyl)butoxy]-1(2H)-isoquinoline, on the survival of TSC2(-) cells. We identified the up-regulation of PARP1 in TSC2(-) cells relative to cells in which wild-type TSC2 has been reintroduced (TSC2-addback [TSC2(+)] cells). The transcript levels of PARP1 in TSC2(-) cells were not affected by rapamycin. PARP1 levels were increased in TSC2(-) cells, xenograft tumors of rat-derived TSC2(-) cells, renal cystadenomas from Tsc2(+/-) mice, and human LAM nodules. RNA interference of mTOR failed to reduce PARP1 levels. Proliferation and survival of TSC2(-) cells was reduced in response to PARP1 inhibitor treatment, more so than TSC2(+) cells. TSC2(-) cells exhibit higher levels of PARP1 relative to TSC2(+) cells in an mTOR-insensitive manner. PARP1 inhibitors selectively suppress the growth and induce apoptosis of TSC2(-) cells from patients with LAM. Targeting PARP1 may be beneficial in the treatment of LAM and other neoplasm with mTORC1 activation.
PARP-1 inhibition induces a late increase in the level of reactive oxygen species in cells after ionizing radiation.[Pubmed:22321899]
Mutat Res. 2012 Apr 1;732(1-2):9-15.
Poly(ADP-ribose) polymerase 1 (PARP1), an enzyme activated by DNA strand breaks, synthesizes polymers of poly(ADP-ribose) (PAR) that modify chromatin and other proteins and play a role in DNA repair. Inhibition of PARP1 activity is considered a potentially important strategy in clinical practice, especially to sensitize tumor cells to chemo- and radio-therapy. Here we examined the influence of inhibition of PARP1 on formation of reactive oxygen species (ROS) and on DNA repair in cells exposed to ionizing radiation (IR). K562 (human myelogenous leukaemia) cells were grown and exposed to 4 or 12 Gy of ionizing radiation in presence or absence of the PARP inhibitor NU1025 (100 muM). Intracellular ROS were assayed using the probe 2,7-dichlorofluorescein with detection by flow cytometry and the rejoining of DNA strand breaks were followed by alkaline single cell gel electrophoresis (comet) assays. In untreated cells a significant increase in PAR formation occurred during the first 5 min after IR, followed by a gradual decrease up to 30 min. Addition of a PARP inhibitor arrested the production of PAR almost completely and decreased the rate of rejoining of DNA strand breaks significantly; however, 3h after irradiation we observed no difference in the amount of DNA strand breaks between PARP inhibitor-treated and untreated cells. Twelve to 48 h after irradiation, an increase of ROS concentration was observed in irradiated cells and ROS levels in PARP inhibitor-treated cells were significantly higher than in cells without inhibitor. Irradiated cells grown in the presence or absence of PARP inhibitor did not differ in the frequencies of apoptotic and necrotic cells or in the activity of caspases at 24, 48 and 72 h after irradiation. Poly(ADP-ribosylation) and inhibition of PARP1 appeared to modulate DNA strand break rejoining and influence the concentration of ROS in irradiated cells.
Differential Potential of Pharmacological PARP Inhibitors for Inhibiting Cell Proliferation and Inducing Apoptosis in Human Breast Cancer Cells.[Pubmed:25981734]
J Cell Biochem. 2015 Dec;116(12):2824-39.
BRCA1/2-mutant cells are hypersensitive to inactivation of poly(ADP-ribose) polymerase 1 (PARP-1). We recently showed that inhibition of PARP-1 by NU1025 is strongly cytotoxic for BRCA1-positive BT-20 cells, but not BRCA1-deficient SKBr-3 cells. These results raised the possibility that other PARP-1 inhibitors, particularly those tested in clinical trials, may be more efficacious against BRCA1-deficient SKBr-3 breast cancer cells than NU1025. Thus, in the presented study the cytotoxicity of four PARP inhibitors under clinical evaluation (olaparib, rucaparib, iniparib and AZD2461) was examined and compared to that of NU1025. The sensitivity of breast cancer cells to the PARP-1 inhibition strongly varied. Remarkably, BRCA-1-deficient SKBr-3 cells were almost completely insensitive to NU1025, olaparib and rucaparib, whereas BRCA1-expressing BT-20 cells were strongly affected by NU1025 even at low doses. In contrast, iniparib and AZD2461 were cytotoxic for both BT-20 and SKBr-3 cells. Of the four tested PARP-1 inhibitors only AZD2461 strongly affected cell cycle progression. Interestingly, the anti-proliferative and pro-apoptotic potential of the tested PARP-1 inhibitors clearly correlated with their capacity to damage DNA. Further analyses revealed that proteomic signatures of the two studied breast cancer cell lines strongly differ, and a set of 197 proteins was differentially expressed in NU1025-treated BT-20 cancer cells. These results indicate that BT-20 cells may harbor an unknown defect in DNA repair pathway(s) rendering them sensitive to PARP-1 inhibition. They also imply that therapeutic applicability of PARP-1 inhibitors is not limited to BRCA mutation carriers but can be extended to patients harboring deficiencies in other components of the pathway(s).
Effect of p53 activity on the sensitivity of human glioblastoma cells to PARP-1 inhibitor in combination with topoisomerase I inhibitor or radiation.[Pubmed:25182801]
Cytometry A. 2014 Nov;85(11):953-61.
Poly (ADP-Ribose) polymerase-1 (PARP-1) is involved in the DNA repairing system by sensing and signaling the presence of DNA damage. Inhibition of PARP-1 is tested in combination with DNA damaging agents such as topoisomerase I inhibitors or ionizing radiations (RT) for the treatment of glioblastoma (GBM). Disruption of p53, widely prevalent in GBMs, plays a major role in DNA repairing system. The current study investigates whether p53 activity has an effect on the sensitivity of human GBM cells to PARP-1 inhibitors in combination with topoisomerase I inhibitor topotecan (TPT) and/or RT. Human GBM cell lines carrying a different functional status of p53 were treated with PARP-1 inhibitor NU1025, in combination with TPT and/or RT. Cytotoxic effects were examined by analyzing the antiproliferative activity, the cell cycle perturbations, and the DNA damage induced by combined treatments. PARP inhibition enhanced the antiproliferative activity, the cell cycle perturbations and the DNA damage induced by both TPT or RT in GBM cells. These effects were influenced by the p53 activity: cells carrying an active p53 were more sensitive to the combination of PARP inhibitor and RT, while cells carrying an inactive p53 displayed a higher sensitivity to the combination of PARP inhibitor and TPT. Our study suggests that p53 activity influences the differential sensitivity of GBM cells to combined treatments of TPT, RT, and PARP inhibitors. (c) 2014 International Society for Advancement of Cytometry.
Insights into the binding of PARP inhibitors to the catalytic domain of human tankyrase-2.[Pubmed:25286857]
Acta Crystallogr D Biol Crystallogr. 2014 Oct;70(Pt 10):2740-53.
The poly(ADP-ribose) polymerase (PARP) family represents a new class of therapeutic targets with diverse potential disease indications. PARP1 and PARP2 inhibitors have been developed for breast and ovarian tumors manifesting double-stranded DNA-repair defects, whereas tankyrase 1 and 2 (TNKS1 and TNKS2, also known as PARP5a and PARP5b, respectively) inhibitors have been developed for tumors with elevated beta-catenin activity. As the clinical relevance of PARP inhibitors continues to be actively explored, there is heightened interest in the design of selective inhibitors based on the detailed structural features of how small-molecule inhibitors bind to each of the PARP family members. Here, the high-resolution crystal structures of the human TNKS2 PARP domain in complex with 16 various PARP inhibitors are reported, including the compounds BSI-201, AZD-2281 and ABT-888, which are currently in Phase 2 or 3 clinical trials. These structures provide insight into the inhibitor-binding modes for the tankyrase PARP domain and valuable information to guide the rational design of future tankyrase-specific inhibitors.
Potentiation of temozolomide and topotecan growth inhibition and cytotoxicity by novel poly(adenosine diphosphoribose) polymerase inhibitors in a panel of human tumor cell lines.[Pubmed:10914735]
Clin Cancer Res. 2000 Jul;6(7):2860-7.
Potent poly(ADP-ribose) polymerase (PARP) inhibitors have been developed that potentiate the cytotoxicity of ionizing radiation and anticancer drugs. The biological effects of two novel PARP inhibitors, NU1025 (8-hydroxy-2-methylquinazolin-4-[3H]one, Ki = 48 nM) and NU1085 [2-(4-hydroxyphenyl)benzamidazole-4-carboxamide, Ki = 6 nM], in combination with temozolomide (TM) or topotecan (TP) have been studied in 12 human tumor cell lines (lung, colon, ovary, and breast cancer). Cells were treated with increasing concentrations of TM or TP +/- NU1025 (50, 200 microM) or NU1085 (10 microM) for 72 h. The potentiation of growth inhibition by NU1025 and NU1085 varied between the cell lines from 1.5- to 4-fold for TM and 1- to 5-fold for TP and was unaffected by p53 status. Clonogenic assays undertaken in two of the cell lines confirmed that the potentiation of growth inhibition reflected the potentiation of cytotoxicity. NU1025 (50 microM) was about as effective as 10 microM NU1085 at potentiating growth inhibition and cytotoxicity, consistent with the relative potencies of the two molecules as PARP inhibitors. Potentiation of cytotoxicity was obtained at concentrations of NU1025 and NU1085 that were not toxic per se; however, NU1085 alone was 3-fold more cytotoxic (LC50 values ranged from 83 to 94 microM) than NU1025 alone (LC50 > 900 microM). These data demonstrate that PARP inhibitors are effective resistance-modifying agents in human tumor cell lines and have provided a comprehensive assessment protocol for the selection of optimum combinations of anticancer drugs, PARP inhibitors, and cell lines for in vivo studies.
Potentiation of anti-cancer agent cytotoxicity by the potent poly(ADP-ribose) polymerase inhibitors NU1025 and NU1064.[Pubmed:9823965]
Br J Cancer. 1998 Nov;78(10):1269-77.
The ability of the potent poly(ADP-ribose) polymerase (PARP) inhibitor, NU1025 (8-hydroxy-2-methyl-quinazolin-4-[3H]one) to potentiate the cytotoxicity of a panel of mechanistically diverse anti-cancer agents was evaluated in L1210 cells. NU1025 enhanced the cytotoxicity of the DNA-methylating agent MTIC, gamma-irradiation and bleomycin 3.5-, 1.4- and 2-fold respectively. The cytotoxicities of the thymidylate synthase inhibitor, nolatrexed, and the cytotoxic nucleoside, gemcitabine, were not increased. Potentiation of MTIC cytotoxicity by a delayed exposure to NU1025 was equally effective as by a simultaneous exposure to NU1025, indicating that the effects of NU1025 were mediated by an inhibition of the cellular recovery. The recovery from potentially lethal gamma-irradiation damage cytotoxicity in plateau-phase cells was also inhibited by NU1025. Investigation of DNA strand breakage and repair in gamma-irradiated cells by alkaline elution demonstrated that NU1025 caused a marked retardation of DNA repair. A structurally different PARP inhibitor, NU1064 (2-methylbenzimidazole-4-carboxamide), also potentiated the cytotoxicity of MTIC, to a similar extent to NU1025. NU1064 potentiated a sublethal concentration of a DNA methylating agent in a concentration-dependent manner. Collectively, these data suggest that the most suitable cytotoxic agents for use in combination with PARP inhibitors are methylating agents, bleomycin and ionizing radiation, but not anti-metabolites.
Resistance-modifying agents. 5. Synthesis and biological properties of quinazolinone inhibitors of the DNA repair enzyme poly(ADP-ribose) polymerase (PARP).[Pubmed:9857092]
J Med Chem. 1998 Dec 17;41(26):5247-56.
Clinical studies concerning the role of poly(ADP-ribose) polymerase (PARP) in the repair of drug- and radiation-induced DNA damage have been impeded by the poor solubility, lack of potency, and limited specificity of currently available inhibitors. A series of 2-alkyl- and 2-aryl-substituted 8-hydroxy-, 8-methoxy-, and 8-methylquinazolin-4(3H)-ones has been synthesized and evaluated for PARP inhibitory activity in permeabilized L1210 murine leukemia cells. 8-Methoxy- and 8-methylquinazolinones (14-34) were readily prepared by acylation of 3-substituted anthranilamides with the appropriate acid chloride, followed by base-catalyzed cyclization. The requisite 8-hydroxyquinazolinones (6, 35-39) were synthesized by demethylation of the corresponding 8-methoxyquinazolinones with BBr3. N-Methylation of 8-methoxy-2-methylquinazolinone (15) with MeI, followed by O-demethylation by BBr3, afforded the control N3-methylquinazolinones 42 and 43, respectively. In general, an 8-hydroxy or 8-methyl substituent enhanced inhibitory activity in comparison with an 8-methoxy group. 2-Phenylquinazolinones were marginally less potent than the corresponding 2-methylquinazolinones, but the introduction of an electron-withdrawing or electron-donating 4'-substituent on the 2-aryl ring invariably increased potency. This was particularly evident in the 8-methylquinazolinone series (IC50 values 0.13-0.27 microM), which are among the most potent PARP inhibitors reported to date. N3-Methylquinazolinones 42 and 43 were essentially devoid of activity (IC50 values > 100 microM). In studies with L1210 cells in vitro, a concentration of 200 microM 8-hydroxy-2-methylquinazolinone (6, NU1025) (IC50 value 0.40 microM) potentiated the cytotoxicity of the monomethylating agent 5-(3-methyltriazen-1-yl)imidazole-4-carboxamide and gamma-radiation 3.5- and 1.4-fold, respectively, at the 10% survival level.


