Erlotinib mesylateEGFR inhibitor CAS# 248594-19-6 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 248594-19-6 | SDF | Download SDF |
| PubChem ID | 9913428 | Appearance | Powder |
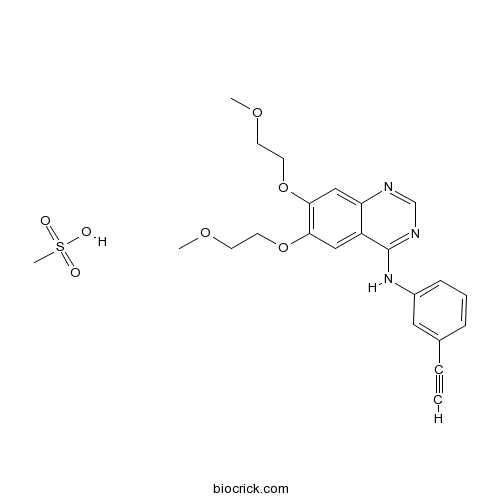
| Formula | C23H27N3O7S | M.Wt | 489.54 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | CP-358774; OSI-774; NSC 718781; R 1415 | ||
| Solubility | 25℃: DMSO | ||
| Chemical Name | N-(3-ethynylphenyl)-6,7-bis(2-methoxyethoxy)quinazolin-4-amine;methanesulfonic acid | ||
| SMILES | COCCOC1=C(C=C2C(=C1)C(=NC=N2)NC3=CC=CC(=C3)C#C)OCCOC.CS(=O)(=O)O | ||
| Standard InChIKey | PCBNMUVSOAYYIH-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C22H23N3O4.CH4O3S/c1-4-16-6-5-7-17(12-16)25-22-18-13-20(28-10-8-26-2)21(29-11-9-27-3)14-19(18)23-15-24-22;1-5(2,3)4/h1,5-7,12-15H,8-11H2,2-3H3,(H,23,24,25);1H3,(H,2,3,4) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Erlotinib mesylate inhibits purified EGFR kinase with an IC50 of 2 nM.In Vitro:Erlotinib (CP-358,774) is also a potent inhibitor of the recombinant intracellular (kinase) domain of the EGFR, with an IC50 of 1 nM. The proliferation of DiFi cells is strongly inhibited by Erlotinib with an IC50 of 100 nM for an 8-day proliferation assay[1]. The combination of B-DIM and Erlotinib (2 μM) results in a significant inhibition of colony formation in BxPC-3 cells when compared with either agent alone. The combination of B-DIM and Erlotinib (2 μM) results in a significant induction of apoptosis only in BxPC-3 cells when compare with the apoptotic effect of either agent alone[2].In Vivo:There is a 1.49-fold statistically significant difference between AUC0-inf after p.o. administration of Erlotinib (5 mg/kg) comparing Bcrp1/Mdr1a/1b-/- and WT mice (7,419±1,720 versus 4,957±1,735 ng*h/mL respectively, P=0.01)[3]. The administration of Erlotinib (10 mg/kg/day, or 20 mg/kg/day) to Bleomycin (BLM)-treated rats shows no exacerbation of lung injuries in indices such as macroscopic findings, lung weights, histopathological scores (lung lesion density and lung fibrosis score), and pulmonary hydroxyproline (HyP) level. The result suggests that Erlotinib does not have any exacerbating effects on lung injuries induced by BLM in rats[4]. References: | |||||

Erlotinib mesylate Dilution Calculator

Erlotinib mesylate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.0427 mL | 10.2137 mL | 20.4273 mL | 40.8547 mL | 51.0683 mL |
| 5 mM | 0.4085 mL | 2.0427 mL | 4.0855 mL | 8.1709 mL | 10.2137 mL |
| 10 mM | 0.2043 mL | 1.0214 mL | 2.0427 mL | 4.0855 mL | 5.1068 mL |
| 50 mM | 0.0409 mL | 0.2043 mL | 0.4085 mL | 0.8171 mL | 1.0214 mL |
| 100 mM | 0.0204 mL | 0.1021 mL | 0.2043 mL | 0.4085 mL | 0.5107 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Erlotinib mesylate is an oral selective inhibitor of epidermal growth factor receptor (EGFR) tyrosine kinase withIC50 value of 20 nM 1,2.
Erlotinib is an inhibitor targeting EGFR, it has been approved by FDA for the treatment of non-small-cell lung cancer (NSCLC), colorectal cancer and head and neck cancer. Erlotinib can bind to the ATP-binding site of EGFR reversibly and inhibit the autophosphorylation completely. This inhibition results in a subsequent inhibition of the downstream signal transduction pathways and causes cell cycle arrest and angiogenesis suppression. Besides that, Erlotinib also showed inhibitory activities against HER1 and HER2 kinases with IC50 values of 20-30 nM and 2-3 µM, respectively 1.
References:
1. Iyer R, Bharthuar A. A review of erlotinib-an oral, selective epidermal growth factor receptor tyrosine kinase inhibitor. Expert opinion on pharmacotherapy, 2010, 11(2): 311-320.
2. Philip P A, Mahoney M R, Allmer C, et al. Phase II study of Erlotinib (OSI-774) in patients with advanced hepatocellular cancer. Journal of Clinical Oncology, 2005, 23(27): 6657-6663.
- Azaphen
Catalog No.:BCC1390
CAS No.:24853-80-3
- Boc-Lys(Boc)-OH
Catalog No.:BCC3412
CAS No.:2483-46-7
- Laquinimod (ABR-215062)
Catalog No.:BCC3802
CAS No.:248281-84-7
- Agmatine sulfate
Catalog No.:BCC6813
CAS No.:2482-00-0
- Reserpinine
Catalog No.:BCN3490
CAS No.:24815-24-5
- H-Cys(tBu)-OH.HCl
Catalog No.:BCC2910
CAS No.:2481-09-6
- (-)-Epiafzelechin
Catalog No.:BCN5114
CAS No.:24808-04-6
- Boc-Orn(Z)-OH
Catalog No.:BCC3430
CAS No.:2480-93-5
- N2-Methyl-L-arginine
Catalog No.:BCC6032
CAS No.:2480-28-6
- H-N-Me-Ser-OH.HCl
Catalog No.:BCC3352
CAS No.:2480-26-4
- N-Me-Val-OH.HCl
Catalog No.:BCC2612
CAS No.:2480-23-1
- Estriol 3-glucuronide
Catalog No.:BCN2239
CAS No.:2479-91-6
- Boc-Met-OH
Catalog No.:BCC3424
CAS No.:2488-15-5
- Mesembrine
Catalog No.:BCN3668
CAS No.:24880-43-1
- Bakkenolide III
Catalog No.:BCN7245
CAS No.:24909-95-3
- H-Met-OMe. HCl
Catalog No.:BCC2995
CAS No.:2491-18-1
- H-Ala-OMe.HCl
Catalog No.:BCC3192
CAS No.:2491-20-5
- beta-Isosparteine
Catalog No.:BCN2326
CAS No.:24915-04-6
- Sarracine
Catalog No.:BCN2021
CAS No.:2492-09-3
- Ethyl 3-cyclopropyl-3-oxopropanoate
Catalog No.:BCC8974
CAS No.:24922-02-9
- Varenicline
Catalog No.:BCC4155
CAS No.:249296-44-4
- Poly(I:C)
Catalog No.:BCC6138
CAS No.:24939-03-5
- Scutebarbatine N
Catalog No.:BCN8385
CAS No.:960302-87-8
- Demethoxy-3-epifumitremorgin C
Catalog No.:BCC8315
CAS No.:106292-68-6
Randomized phase II study of two intercalated combinations of eribulin mesylate and erlotinib in patients with previously treated advanced non-small-cell lung cancer.[Pubmed:24827127]
Ann Oncol. 2014 Aug;25(8):1578-84.
BACKGROUND: This phase II, open-label study investigated intercalated combinations of eribulin and erlotinib in unselected patients with advanced non-small-cell lung cancer previously treated with platinum-based chemotherapies. PATIENTS AND METHODS: Eligible patients were randomized to eribulin mesylate 2.0 mg/m(2) on day 1 with erlotinib 150 mg on days 2-16 (21-day regimen) or eribulin mesylate 1.4 mg/m(2) on days 1 and 8 with erlotinib 150 mg on days 15-28 (28-day regimen). The primary end point was objective response rate (ORR). RESULTS: One hundred and twenty-three patients received >/= 1 cycle of therapy (63, 21-day regimen; 60, 28-day regimen). ORRs were 13% [95% confidence interval (CI) 6%-24%] and 17% (95% CI 8%-29%), and disease control rates were 48% (95% CI 35%-61%) and 63% (95% CI 50%-75%) for the 21- and 28-day regimens, respectively. The median progression-free survival and overall survival were similar with both regimens. Both regimens were well tolerated with common grade >/= 3 toxicities being neutropenia, asthenia/fatigue, and dyspnoea. Sequential administration of erlotinib did not interfere with the pharmacokinetic profile of eribulin. CONCLUSION: Intercalated combination of eribulin and erlotinib demonstrated modest activity and the addition of erlotinib did not appear to improve treatment outcome in an unselected population. The 28-day regimen is suitable for further investigation. Clinicaltrials.gov identifier: NCT01104155.


