Laquinimod (ABR-215062)Immunomodulator,orally-available,quinolinone-based and broad spectrum CAS# 248281-84-7 |
- CUDC-101
Catalog No.:BCC2149
CAS No.:1012054-59-9
- Valproic acid sodium salt (Sodium valproate)
Catalog No.:BCC2156
CAS No.:1069-66-5
- M344
Catalog No.:BCC2162
CAS No.:251456-60-7
- Belinostat (PXD101)
Catalog No.:BCC2153
CAS No.:414864-00-9
- Trichostatin A (TSA)
Catalog No.:BCC3605
CAS No.:58880-19-6
- JNJ-26481585
Catalog No.:BCC2147
CAS No.:875320-29-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 248281-84-7 | SDF | Download SDF |
| PubChem ID | 54677946 | Appearance | Powder |
| Formula | C19H17ClN2O3 | M.Wt | 356.8 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | ABR-215062 | ||
| Solubility | DMSO : 100 mg/mL (280.27 mM; Need ultrasonic) | ||
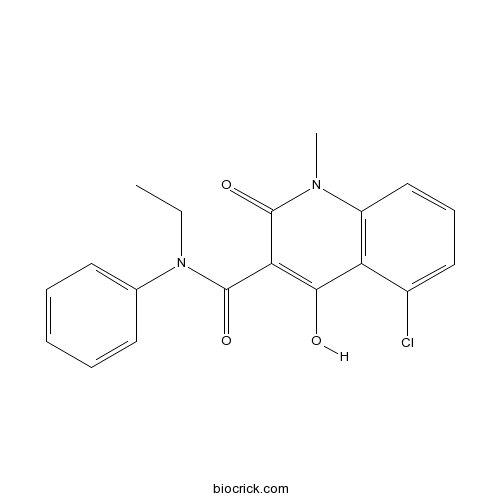
| Chemical Name | 5-chloro-N-ethyl-4-hydroxy-1-methyl-2-oxo-N-phenylquinoline-3-carboxamide | ||
| SMILES | CCN(C1=CC=CC=C1)C(=O)C2=C(C3=C(C=CC=C3Cl)N(C2=O)C)O | ||
| Standard InChIKey | GKWPCEFFIHSJOE-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C19H17ClN2O3/c1-3-22(12-8-5-4-6-9-12)19(25)16-17(23)15-13(20)10-7-11-14(15)21(2)18(16)24/h4-11,23H,3H2,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Laquinimod is an orally-available. quinolinone-based, broad spectrum immunomodulator with anti-inflammatory activity |

Laquinimod (ABR-215062) Dilution Calculator

Laquinimod (ABR-215062) Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.8027 mL | 14.0135 mL | 28.0269 mL | 56.0538 mL | 70.0673 mL |
| 5 mM | 0.5605 mL | 2.8027 mL | 5.6054 mL | 11.2108 mL | 14.0135 mL |
| 10 mM | 0.2803 mL | 1.4013 mL | 2.8027 mL | 5.6054 mL | 7.0067 mL |
| 50 mM | 0.0561 mL | 0.2803 mL | 0.5605 mL | 1.1211 mL | 1.4013 mL |
| 100 mM | 0.028 mL | 0.1401 mL | 0.2803 mL | 0.5605 mL | 0.7007 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
ABR-215062 is an orally active immunoregulator. Experimental autoimmune encephalomyelitis (EAE) is an inflammatory autoimmune disease of the CNS that can be elicited in rodents and represents the major animal model for the study of multiple sclerosis (MS).
In vitro: ABR-215062 was shown to completely inhibit the development of murine acute experimental autoimmune encephalomyelitis (EAE) [1].
In vivo: ABR-215062 dose-dependently inhibited disease and showed better disease inhibitory effects as compared to roquinimex (Linomide). Furthermore, ABR-215062 inhibited the inflammation of both CD4+ T cells and macrophages into central nervous tissues [2].
Clinical trial: Randomized, controlled clinical trials in relapsing MS demonstrate a dose–response effect of ABR-215062 on disease activities, indicated by reduced clinical relapse rate, number of brain MRI active lesions, and sustained disability and brain atrophy [3].
References:
[1] Brunmark C, Runström A, Ohlsson L, Sparre B, Brodin T, Aström M, Hedlund G. The new orally active immunoregulator laquinimod (ABR-215062) effectively inhibits development and relapses of experimental autoimmune encephalomyelitis. J Neuroimmunol. 2002 Sep;130(1-2):163-72.
[2] Yang JS, Xu LY, Xiao BG, Hedlund G, Link H. Laquinimod (ABR-215062) suppresses the development of experimental autoimmune encephalomyelitis, modulates the Th1/Th2 balance and induces the Th3 cytokine TGF-beta in Lewis rats. J Neuroimmunol. 2004 Nov;156(1-2):3-9.
[3] Haggiag S, Ruggieri S, Gasperini C. Efficacy and safety of laquinimod in multiple sclerosis: current status. Ther Adv Neurol Disord. 2013 Nov;6(6):343-52.
- Agmatine sulfate
Catalog No.:BCC6813
CAS No.:2482-00-0
- Reserpinine
Catalog No.:BCN3490
CAS No.:24815-24-5
- H-Cys(tBu)-OH.HCl
Catalog No.:BCC2910
CAS No.:2481-09-6
- (-)-Epiafzelechin
Catalog No.:BCN5114
CAS No.:24808-04-6
- Boc-Orn(Z)-OH
Catalog No.:BCC3430
CAS No.:2480-93-5
- N2-Methyl-L-arginine
Catalog No.:BCC6032
CAS No.:2480-28-6
- H-N-Me-Ser-OH.HCl
Catalog No.:BCC3352
CAS No.:2480-26-4
- N-Me-Val-OH.HCl
Catalog No.:BCC2612
CAS No.:2480-23-1
- Estriol 3-glucuronide
Catalog No.:BCN2239
CAS No.:2479-91-6
- 1,3-Bis(4-aminophenoxy)benzene
Catalog No.:BCC8418
CAS No.:2479-46-1
- Beta-Peltoboykinolic acid
Catalog No.:BCN6635
CAS No.:24778-48-1
- SB 328437
Catalog No.:BCC6056
CAS No.:247580-43-4
- Boc-Lys(Boc)-OH
Catalog No.:BCC3412
CAS No.:2483-46-7
- Azaphen
Catalog No.:BCC1390
CAS No.:24853-80-3
- Erlotinib mesylate
Catalog No.:BCC1558
CAS No.:248594-19-6
- Boc-Met-OH
Catalog No.:BCC3424
CAS No.:2488-15-5
- Mesembrine
Catalog No.:BCN3668
CAS No.:24880-43-1
- Bakkenolide III
Catalog No.:BCN7245
CAS No.:24909-95-3
- H-Met-OMe. HCl
Catalog No.:BCC2995
CAS No.:2491-18-1
- H-Ala-OMe.HCl
Catalog No.:BCC3192
CAS No.:2491-20-5
- beta-Isosparteine
Catalog No.:BCN2326
CAS No.:24915-04-6
- Sarracine
Catalog No.:BCN2021
CAS No.:2492-09-3
- Ethyl 3-cyclopropyl-3-oxopropanoate
Catalog No.:BCC8974
CAS No.:24922-02-9
- Varenicline
Catalog No.:BCC4155
CAS No.:249296-44-4
Laquinimod (ABR-215062) for the treatment of relapsing multiple sclerosis.[Pubmed:26536299]
Expert Rev Clin Pharmacol. 2016;9(1):49-57.
Laquinimod (ABR-215062) is an oral immunomodulatory agent developed for the treatment of relapsing multiple sclerosis (MS). Laquinimod is a derivative of the drug roquinimex, but lacks the unacceptable side effect profile of this drug which was documented in previous MS trials. Preclinical studies in experimental models of MS, both of autoimmune neuroinflammation and of toxin induced demyelination show a multitude of immunomodulatory and anti-inflammatory effects, including some that are effective directly in the central nervous system. Phase I study results confirmed the safety and tolerability of laquinimod, and phase II and III studies provide a picture of a consistent albeit moderate effect on relapse rate and new lesion development on magnetic resonance imaging combined with a stronger effect on sustained progression and brain atrophy. These findings make laquinimod a potentially useful future treatment of MS.
The new orally active immunoregulator laquinimod (ABR-215062) effectively inhibits development and relapses of experimental autoimmune encephalomyelitis.[Pubmed:12225898]
J Neuroimmunol. 2002 Sep;130(1-2):163-72.
A new orally active drug, Laquinimod (ABR-215062), was shown to completely inhibit the development of murine acute experimental autoimmune encephalomyelitis (EAE). Furthermore, leukocyte infiltration into the central nervous system (CNS) was abolished in the laquinimod-treated animals. By direct comparison based on dose and total exposure, laquinimod was approximately 20 times more potent than the immunomodulator roquinimex. Laquinimod also had clear therapeutic effect when given after clinical onset in a chronic relapsing EAE model. It therefore represents a new orally active immunoregulatory drug without general immunosuppressive properties for the treatment of the autoimmune disease multiple sclerosis.
Laquinimod (ABR-215062) suppresses the development of experimental autoimmune encephalomyelitis, modulates the Th1/Th2 balance and induces the Th3 cytokine TGF-beta in Lewis rats.[Pubmed:15465591]
J Neuroimmunol. 2004 Nov;156(1-2):3-9.
The new orally active drug Laquinimod (ABR-215062) was evaluated in experimental autoimmune encephalomyelitis (EAE) in the Lewis rat. EAE shares important immunological and clinical features with multiple sclerosis (MS). Doses of 16, 1.6 and 0.16 mg/kg/day laquinimod dose-dependently inhibited disease and showed better disease inhibitory effects as compared to roquinimex (Linomide). Furthermore, laquinimod inhibited the inflammation of both CD4+ T cells and macrophages into central nervous tissues, i.e. the spinal cord. It also changed the cytokine balance in favour of TH2/TH3 cytokines IL-4, IL-10 and TGF-beta. Laquinimod therefore represents a new orally active immunoregulatory drug without general immunosuppressive properties with a potential for the treatment of severe autoimmune diseases like MS.
Inhibition of the development of chronic experimental autoimmune encephalomyelitis by laquinimod (ABR-215062) in IFN-beta k.o. and wild type mice.[Pubmed:16472873]
J Neuroimmunol. 2006 Apr;173(1-2):69-78.
Laquinimod is a novel oral immunomodulatory substance, which is currently developed for the treatment of multiple sclerosis (MS). The ability of laquinimod to inhibit disease development was investigated in chronic experimental autoimmune encephalomyelitis (chEAE) in IFN-beta k.o. mice and wild type mice. Laquinimod was shown to inhibit both disease development and histopathological changes in the CNS. Furthermore, laquinimod was found to be independent of endogenous IFN-beta for its effect in chEAE. When laquinimod was combined with exogenous IFN-beta, a synergistic disease inhibitory effect was seen. These findings using laquinimod in preclinical disease models for MS emphasize the potential of laquinimod in the future treatment of MS also in patients that do not respond to IFN-beta monotherapy. Furthermore, the results indicate that laquinimod may favourably be combined with IFN-beta.


