Gomisin OCAS# 72960-22-6 |
- Epigomisin O
Catalog No.:BCN2862
CAS No.:73036-31-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 72960-22-6 | SDF | Download SDF |
| PubChem ID | 5317808 | Appearance | Powder |
| Formula | C23H28O7 | M.Wt | 416.46 |
| Type of Compound | Lignans | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
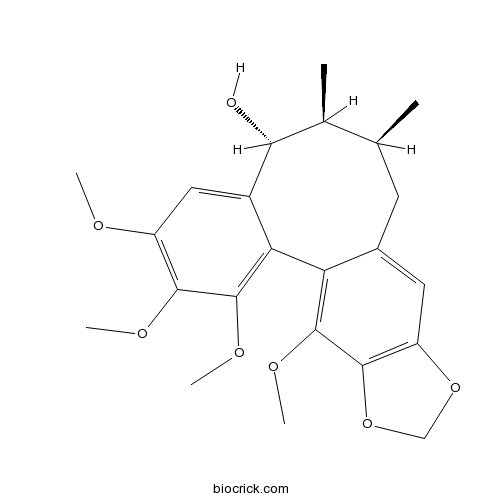
| Chemical Name | (8R,9S,10S)-3,4,5,19-tetramethoxy-9,10-dimethyl-15,17-dioxatetracyclo[10.7.0.02,7.014,18]nonadeca-1(19),2,4,6,12,14(18)-hexaen-8-ol | ||
| SMILES | CC1CC2=CC3=C(C(=C2C4=C(C(=C(C=C4C(C1C)O)OC)OC)OC)OC)OCO3 | ||
| Standard InChIKey | GWDFJIBHVSYXQL-SYTFOFBDSA-N | ||
| Standard InChI | InChI=1S/C23H28O7/c1-11-7-13-8-16-21(30-10-29-16)22(27-5)17(13)18-14(19(24)12(11)2)9-15(25-3)20(26-4)23(18)28-6/h8-9,11-12,19,24H,7,10H2,1-6H3/t11-,12-,19+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Gomisin O is a natural product from Schizandra chinensis. |
| Structure Identification | Chemical & pharmaceutical bulletin 27(11), 2695-2709, 1979-11-25The Constituents of Schizandra chinensis BAILL. V. The Structures of Four New Lignans, Gomisin N, Gomisin O, Epigomisin O and Gomisin E, and Transformation of Gomisin N to Deangeloylgomisin B[Reference: WebLink]Four new dibenzocyclooctadiene lignans, gomisin N (1), Gomisin O (2) and gomisin E (4), and epiGomisin O (3), together with a known lignan (+)-deoxyschizandrin (5) were isolated from the fruits of Schizandra chinensis BAILL. (Schizandraceae).
J Org Chem. 2005 Oct 28;70(22):8932-41.Asymmetric total synthesis of dibenzocyclooctadiene lignan natural products.[Pubmed: 16238330 ]Full details of the asymmetric total syntheses of the dibenzocyclooctadiene lignans interiotherin A, angeloylgomisin R, Gomisin O, and gomisin E (epiGomisin O) are presented.
|

Gomisin O Dilution Calculator

Gomisin O Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.4012 mL | 12.006 mL | 24.0119 mL | 48.0238 mL | 60.0298 mL |
| 5 mM | 0.4802 mL | 2.4012 mL | 4.8024 mL | 9.6048 mL | 12.006 mL |
| 10 mM | 0.2401 mL | 1.2006 mL | 2.4012 mL | 4.8024 mL | 6.003 mL |
| 50 mM | 0.048 mL | 0.2401 mL | 0.4802 mL | 0.9605 mL | 1.2006 mL |
| 100 mM | 0.024 mL | 0.1201 mL | 0.2401 mL | 0.4802 mL | 0.6003 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Gomisin E
Catalog No.:BCN7031
CAS No.:72960-21-5
- 6,7-Dihydroneridienone A
Catalog No.:BCN4020
CAS No.:72959-46-7
- 2-Furoyl-LIGRLO-amide
Catalog No.:BCC3958
CAS No.:729589-58-6
- Carvedilol
Catalog No.:BCC4324
CAS No.:72956-09-3
- DL-Catechin
Catalog No.:BCN6325
CAS No.:7295-85-4
- 30-Hydroxylup-20(29)-en-3-one
Catalog No.:BCN4286
CAS No.:72944-06-0
- Octadecyl p-coumarate
Catalog No.:BCN7235
CAS No.:72943-88-5
- K858
Catalog No.:BCC7760
CAS No.:72926-24-0
- Bohemamine
Catalog No.:BCN1958
CAS No.:72926-12-6
- (RS)-4-Carboxyphenylglycine
Catalog No.:BCC6601
CAS No.:7292-81-1
- α,β-Methyleneadenosine 5'-triphosphate trisodium salt
Catalog No.:BCC3591
CAS No.:7292-42-4
- (Z)-Butylidenephthalide
Catalog No.:BCN4007
CAS No.:72917-31-8
- XRP44X
Catalog No.:BCC7568
CAS No.:729605-21-4
- BMS-707035
Catalog No.:BCC2133
CAS No.:729607-74-3
- Brassinolide
Catalog No.:BCC1438
CAS No.:72962-43-7
- L-(-)-threo-3-Hydroxyaspartic acid
Catalog No.:BCC6565
CAS No.:7298-99-9
- Cordycepin
Catalog No.:BCN5389
CAS No.:73-03-0
- H-Trp-OH
Catalog No.:BCC3111
CAS No.:73-22-3
- Adenine
Catalog No.:BCC4450
CAS No.:73-24-5
- Melatonin
Catalog No.:BCN2196
CAS No.:73-31-4
- H-Ile-OH
Catalog No.:BCC2960
CAS No.:73-32-5
- Guanine
Catalog No.:BCN8414
CAS No.:73-40-5
- Lidocaine hydrochloride
Catalog No.:BCC9009
CAS No.:73-78-9
- 15-Isopimarene-8,18-diol
Catalog No.:BCN4287
CAS No.:73002-86-5
Asymmetric total synthesis of dibenzocyclooctadiene lignan natural products.[Pubmed:16238330]
J Org Chem. 2005 Oct 28;70(22):8932-41.
[structure: see text] Full details of the asymmetric total syntheses of the dibenzocyclooctadiene lignans interiotherin A, angeloylgomisin R, Gomisin O, and gomisin E (epiGomisin O) are presented. The syntheses were based on a unified synthetic strategy involving a novel crotylation using the Leighton auxiliary that occurred with excellent asymmetric induction (>98:2 enantiomeric ratio), a diastereoselective hydroboration/Suzuki-Miyaura coupling reaction sequence, and an atropdiastereoselective biarylcuprate coupling, both of which occurred with total (>20:1) stereocontrol. The syntheses were achieved in six to eight steps from simple aromatic precursors.


