H-Lys(Fmoc)-OHCAS# 84624-28-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 84624-28-2 | SDF | Download SDF |
| PubChem ID | 7010541 | Appearance | Powder |
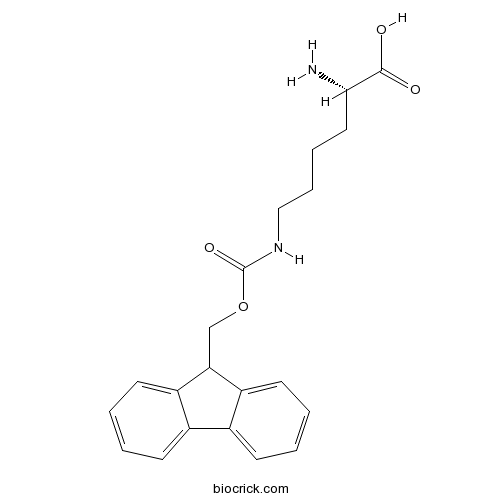
| Formula | C21H24N2O4 | M.Wt | 368.4 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (2S)-2-amino-6-(9H-fluoren-9-ylmethoxycarbonylamino)hexanoic acid | ||
| SMILES | C1=CC=C2C(=C1)C(C3=CC=CC=C32)COC(=O)NCCCCC(C(=O)O)N | ||
| Standard InChIKey | RAQBUPMYCNRBCQ-IBGZPJMESA-N | ||
| Standard InChI | InChI=1S/C21H24N2O4/c22-19(20(24)25)11-5-6-12-23-21(26)27-13-18-16-9-3-1-7-14(16)15-8-2-4-10-17(15)18/h1-4,7-10,18-19H,5-6,11-13,22H2,(H,23,26)(H,24,25)/t19-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

H-Lys(Fmoc)-OH Dilution Calculator

H-Lys(Fmoc)-OH Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.7144 mL | 13.5722 mL | 27.1444 mL | 54.2888 mL | 67.861 mL |
| 5 mM | 0.5429 mL | 2.7144 mL | 5.4289 mL | 10.8578 mL | 13.5722 mL |
| 10 mM | 0.2714 mL | 1.3572 mL | 2.7144 mL | 5.4289 mL | 6.7861 mL |
| 50 mM | 0.0543 mL | 0.2714 mL | 0.5429 mL | 1.0858 mL | 1.3572 mL |
| 100 mM | 0.0271 mL | 0.1357 mL | 0.2714 mL | 0.5429 mL | 0.6786 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
H-Lys(Fmoc)-OH
- Boc-Lys(Fmoc)-OH
Catalog No.:BCC3417
CAS No.:84624-27-1
- Fmoc-D-Val-OH
Catalog No.:BCC3573
CAS No.:84624-17-9
- Cyclogalegigenin
Catalog No.:BCN6295
CAS No.:84605-18-5
- Lorazepam
Catalog No.:BCC5970
CAS No.:846-49-1
- Boldenone
Catalog No.:BCC8892
CAS No.:846-48-0
- 5α-Androstanedione
Catalog No.:BCC8752
CAS No.:846-46-8
- Serrin A
Catalog No.:BCN6985
CAS No.:845959-98-0
- 2-Methyl-5-hydroxytryptamine hydrochloride
Catalog No.:BCC5663
CAS No.:845861-49-6
- CP 94253 hydrochloride
Catalog No.:BCC7018
CAS No.:845861-39-4
- Bakuchalcone
Catalog No.:BCN3201
CAS No.:84575-13-3
- Cleomiscosin C
Catalog No.:BCN4388
CAS No.:84575-10-0
- Rocaglamide
Catalog No.:BCN4387
CAS No.:84573-16-0
- Itraconazole
Catalog No.:BCC4914
CAS No.:84625-61-6
- Eurycomanone
Catalog No.:BCN2990
CAS No.:84633-29-4
- 4-Nitrobenzyl dimethylcarbamate
Catalog No.:BCN3284
CAS No.:84640-31-3
- Tea polyphenol
Catalog No.:BCN8518
CAS No.:84650-60-2
- Decinnamoyltaxinine J
Catalog No.:BCN7210
CAS No.:84652-33-5
- Lorcaserin HCl
Catalog No.:BCC5041
CAS No.:846589-98-8
- Isoastragaloside I
Catalog No.:BCN2979
CAS No.:84676-88-0
- Astragaloside II
Catalog No.:BCN5962
CAS No.:84676-89-1
- Enalaprilat Dihydrate
Catalog No.:BCC5009
CAS No.:84680-54-6
- 3-O-(2'E,4'Z-Decadienoyl)ingenol
Catalog No.:BCN3767
CAS No.:84680-59-1
- Astragaloside I
Catalog No.:BCN5961
CAS No.:84680-75-1
- Astragaloside III
Catalog No.:BCN5963
CAS No.:84687-42-3
The production of anti-hexapeptide antibodies which recognize the S7, L6 and L13 ribosomal proteins of Escherichia coli.[Pubmed:11931584]
J Pept Sci. 2002 Mar;8(3):118-24.
Here we report the synthesis of the N-terminal hexapeptide H-Pro-Arg-Arg-Arg-Val-Ile-OH of the E. coli ribosomal protein S7. the C-terminal hexapeptide H-Lys-Glu-Ala-Lys-Lys-Lys-OH of L6 and the C-terminal hexapeptide H-Pro-Gln-Val-Leu-Asp-Ile-OH of L13. All peptides were prepared by SPPS following the Fmoc-strategy, using DIC/HOBt and/or HBTU as coupling reagents and 2-chlorotrityl chloride resin as the solid support. The carrier linked synthetic peptides were injected into rabbits and elicited an anti-peptide response. These anti-hexapeptide antibodies were found to recognize the corresponding peptides and proteins.
Synthesis of glycosylated tuftsins and tuftsin-containing IgG fragment undecapeptide.[Pubmed:2019473]
Int J Pept Protein Res. 1991 Feb;37(2):112-21.
Syntheses are described of two new tuftsin derivatives containing a 2-acetamido-2-deoxy-D-galactopyranosyl unit alpha- or beta-glycosidically linked to the threonine's hydroxy side chain function and of the glycosylated undecapeptide corresponding to the tuftsin region of the heavy chain of IgG (amino acid sequence 289-299). The glycosylated tuftsins were synthesized by the solution procedure. Fmoc-[Gal NAc(Ac)3 alpha]Thr-OH and Fmoc-[GalNAc(Ac)3 beta]Thr-OH were allowed to react with H-Lys(Z)-Pro-Arg(NO2)-OBzl by the mixed anhydride procedure and the resulting glycosylated tetrapeptides were fully deblocked by catalytic hydrogenation followed by treatment with potassium cyanide, purified by ion exchange chromatography and characterized by analytical HPLC, elemental and amino acid analyses, optical rotation, and proton NMR spectroscopy. Synthesis of the glycosylated undecapeptide was achieved by the continuous flow solid phase procedure on 4-hydroxymethylphenoxyacetyl-norleucyl derivatized Kieselguhr-supported resin. Fmoc-amino acid symmetrical anhydrides or pentafluorophenyl esters, in the presence of N-hydroxybenzotriazole, were used as the acylating agents. To mimic the native sequence of the tuftsin region at the Fc-domain of immunoglobulin G a 2-acetamido-2-deoxy-beta-D-glucopyranosyl unit was N-glycosidically linked to the amide side chain of Asn 297. The glycosylated asparagine residue was introduced as N2-fluorenylmethyloxycarbonyl-N4-(2-acetamido-3,4,6-tri-O-acetyl-2 -deoxy-beta-D - glucopyranosyl)-asparagine pentafluorophenyl ester. After cleavage from the resin the glycopeptide was deprotected, purified by ion exchange chromatography, and characterized by analytical HPLC, amino acid analysis, high voltage electrophoresis, and proton NMR. The conformational features of the glyco-undecapeptide were determined by circular dichroism measurements both in water and in 98% trifluoroethanol. Results of biological assays will be published elsewhere.
Synthesis of O-glycosylated tuftsins by utilizing threonine derivatives containing an unprotected monosaccharide moiety.[Pubmed:2119354]
Int J Pept Protein Res. 1990 Jul;36(1):86-96.
Synthesis is described of four tuftsin derivatives containing a D-glucopyranosyl or a D-galactopyranosyl unit covalently linked to the hydroxy side chain function of the threonine residue through either an alpha or beta O-glycosidic linkage. Fmoc-threonine derivatives containing the suitable unprotected sugar were used for incorporating the O-glycosylated amino acid residue. Z-Thr[alpha-Glc(OBzl)4]-OBzl and Z-Thr[alpha-Gal(OBzl)4]-OBzl were prepared from the tetra-O-benzylated sugar and Z-Thr-OBzl by the trichloroacetimidate method in the presence of trimethylsilyl trifluoromethane sulfonate. The alpha glycosylated threonine derivatives were converted into Fmoc-Thr(alpha-Glc)-OH and Fmoc-Thr(alpha-Gal)-OH by catalytic hydrogenation followed by acylation with Fmoc-OSu. beta-Glucosylation and beta-galactosylation of threonine were carried out by reacting the proper per-O-acetylated sugar with Z-Thr-OBzl and boron trifluoride ethyl etherate in dichloromethane. Catalytic hydrogenation of the beta-O-glycosylated threonine derivatives followed by acylation with Fmoc-OSu and deacetylation with methanolic hydrazine yielded Fmoc-Thr(beta-Glc)-OH and Fmoc-Thr(beta-Gal)-OH, respectively. The O-glycosylated threonine derivatives were then reacted with H-Lys(Z)-Pro-Arg(NO2)-OBzl in the presence of DCC and HOBt and the resulting glycosylated tuftsin derivatives were fully deblocked by catalytic hydrogenation, purified by HPLC, and characterized by optical rotation, amino acid analysis, and 1H NMR. The beta-galactosylated tuftsin was also prepared by the continuous flow solid phase procedure.


