LorazepamActs at the benzodiazepine modulatory site CAS# 846-49-1 |
- Bavisant dihydrochloride hydrate
Catalog No.:BCC1404
CAS No.:1103522-80-0
- Lidocaine
Catalog No.:BCC1084
CAS No.:137-58-6
- Mianserin HCl
Catalog No.:BCC1114
CAS No.:21535-47-7
- Bavisant
Catalog No.:BCC1402
CAS No.:929622-08-2
- Bavisant dihydrochloride
Catalog No.:BCC1403
CAS No.:929622-09-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 846-49-1 | SDF | Download SDF |
| PubChem ID | 3958 | Appearance | Powder |
| Formula | C15H10Cl2N2O2 | M.Wt | 321.16 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in DMSO and to 40 mM in ethanol | ||
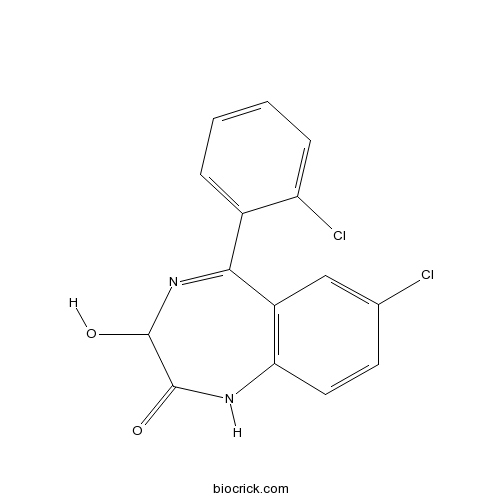
| Chemical Name | 7-chloro-5-(2-chlorophenyl)-3-hydroxy-1,3-dihydro-1,4-benzodiazepin-2-one | ||
| SMILES | C1=CC=C(C(=C1)C2=NC(C(=O)NC3=C2C=C(C=C3)Cl)O)Cl | ||
| Standard InChIKey | DIWRORZWFLOCLC-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C15H10Cl2N2O2/c16-8-5-6-12-10(7-8)13(19-15(21)14(20)18-12)9-3-1-2-4-11(9)17/h1-7,15,21H,(H,18,20) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Ligand at the GABAA receptor benzodiazepine modulatory site. Anxiolytic, anticonvulsant and sedative/hypnotic agent. |

Lorazepam Dilution Calculator

Lorazepam Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.1137 mL | 15.5686 mL | 31.1371 mL | 62.2743 mL | 77.8428 mL |
| 5 mM | 0.6227 mL | 3.1137 mL | 6.2274 mL | 12.4549 mL | 15.5686 mL |
| 10 mM | 0.3114 mL | 1.5569 mL | 3.1137 mL | 6.2274 mL | 7.7843 mL |
| 50 mM | 0.0623 mL | 0.3114 mL | 0.6227 mL | 1.2455 mL | 1.5569 mL |
| 100 mM | 0.0311 mL | 0.1557 mL | 0.3114 mL | 0.6227 mL | 0.7784 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Boldenone
Catalog No.:BCC8892
CAS No.:846-48-0
- 5α-Androstanedione
Catalog No.:BCC8752
CAS No.:846-46-8
- Serrin A
Catalog No.:BCN6985
CAS No.:845959-98-0
- 2-Methyl-5-hydroxytryptamine hydrochloride
Catalog No.:BCC5663
CAS No.:845861-49-6
- CP 94253 hydrochloride
Catalog No.:BCC7018
CAS No.:845861-39-4
- Bakuchalcone
Catalog No.:BCN3201
CAS No.:84575-13-3
- Cleomiscosin C
Catalog No.:BCN4388
CAS No.:84575-10-0
- Rocaglamide
Catalog No.:BCN4387
CAS No.:84573-16-0
- PHA-767491
Catalog No.:BCC1858
CAS No.:845714-00-3
- 4-Hydroxycephalotaxine
Catalog No.:BCN4386
CAS No.:84567-08-8
- Bitopertin (R enantiomer)
Catalog No.:BCC1420
CAS No.:845614-12-2
- Bitopertin
Catalog No.:BCC1419
CAS No.:845614-11-1
- Cyclogalegigenin
Catalog No.:BCN6295
CAS No.:84605-18-5
- Fmoc-D-Val-OH
Catalog No.:BCC3573
CAS No.:84624-17-9
- Boc-Lys(Fmoc)-OH
Catalog No.:BCC3417
CAS No.:84624-27-1
- H-Lys(Fmoc)-OH
Catalog No.:BCC2984
CAS No.:84624-28-2
- Itraconazole
Catalog No.:BCC4914
CAS No.:84625-61-6
- Eurycomanone
Catalog No.:BCN2990
CAS No.:84633-29-4
- 4-Nitrobenzyl dimethylcarbamate
Catalog No.:BCN3284
CAS No.:84640-31-3
- Tea polyphenol
Catalog No.:BCN8518
CAS No.:84650-60-2
- Decinnamoyltaxinine J
Catalog No.:BCN7210
CAS No.:84652-33-5
- Lorcaserin HCl
Catalog No.:BCC5041
CAS No.:846589-98-8
- Isoastragaloside I
Catalog No.:BCN2979
CAS No.:84676-88-0
- Astragaloside II
Catalog No.:BCN5962
CAS No.:84676-89-1
Formulating a poorly water soluble drug into an oral solution suitable for paediatric patients; lorazepam as a model drug.[Pubmed:28126558]
Eur J Pharm Sci. 2017 Mar 30;100:205-210.
INTRODUCTION: Many drugs are unavailable in suitable oral paediatric dosage forms, and pharmacists often have to compound drugs to provide paediatric patients with an acceptable formulation in the right dose. Liquid formulations offer the advantage of dosing flexibility and ease of administration to young patients, but drug substances often show poor aqueous solubility. The objective of this work was to study different solvents and matrices to design a liquid formulation for poorly water soluble drugs, using Lorazepam as model drug. METHODS: Three different formulation strategies were explored to improve the solubility. Firstly, water-soluble organic solvents were used to improve the aqueous solubility directly, secondly, ionic surfactants were used to solubilise the model drug, and thirdly, complexation of Lorazepam with cyclodextrin was studied. Specific attention was paid to excipients, adequate taste correction and palatability. For the final formulation, physical and chemical stability and microbiological quality were assessed for 12months. RESULTS: An organic solvent based formulation, containing a mixture of polyethylene glycol and glycerol 85%, with a minimum amount of propylene glycol, proved to be physically and chemically stable. Development of the non-ionic surfactants formulation was discontinued due to taste problems. The cyclodextrin formulations were physically stable, but Lorazepam content declined to 90% within five months. The final formulation contained in volume concentration (%v/v) 87% glycerol, 10% polyethylene glycol 400 and 3% propylene glycol. Orange essence was the preferred taste corrector. The formulation remained stable for 12months at 4 degrees C, with Lorazepam content remaining >95%. Related substances increased during the study period but remained below 2%. In-use stability was proven up to 4weeks. CONCLUSION: An organic solvent based oral formulation was shown to be superior to a non-ionic surfactant based formulation or a cyclodextrin formulation. These results may help to formulate paediatric formulations of other poorly water soluble drugs, to aid pharmacy compounding.
A novel animal model of acquired human temporal lobe epilepsy based on the simultaneous administration of kainic acid and lorazepam.[Pubmed:28157273]
Epilepsia. 2017 Feb;58(2):222-230.
OBJECTIVE: Kainic acid (KA) is a potent glutamate analog that is used to induce neurodegeneration and model temporal lobe epilepsy (TLE) in rodents. KA reliably induces severe, prolonged seizures, that is, convulsive status epilepticus (cSE), which is typically fatal without pharmacologic intervention. Although the use of KA to model human epilepsy has proven unquestionably valuable for >30 years, significant variability and mortality continue to confound results. These issues are probably the consequence of cSE, an all-or-nothing response that is inherently capricious and uncontrollable. The relevance of cSE to the human condition is dubious, however, as most patients with epilepsy never experienced it. We sought to develop a simple, KA-based animal model of TLE that avoids cSE and its confounds. METHODS: Adult, male Sprague-Dawley rats received coincident subcutaneous injections of KA (5 mg) and Lorazepam (0.25 mg), approximately 15.0 and 0.75 mg/kg, respectively. Continuous video-electroencephalography (EEG) was used to monitor acute seizure activity and detect spontaneous seizures. Immunocytochemistry, Fluoro-Jade B staining, and Timm staining were used to characterize both acute and chronic neuropathology. RESULTS: Acutely, focal hippocampal seizures were induced, which began after about 30 min and were self-terminating after a few hours. Widespread hippocampal neurodegeneration was detected after 4 days. Spontaneous, focal hippocampal seizures began after an average of 12 days in all animals. Classic hippocampal sclerosis and mossy fiber sprouting characterized the long-term neuropathology. Morbidity and mortality rates were both 0%. SIGNIFICANCE: We show here that the effects of systemic KA can be limited to the hippocampus simply with coadministration of a benzodiazepine at a low dose. This means that Lorazepam can block convulsive seizures without truly stopping seizure activity. This novel, cSE-free animal model reliably mimics the defining characteristics of acquired mesial TLE: hippocampal sclerosis and spontaneous hippocampal-onset seizures after a prolonged seizure-free period, without significant morbidity, mortality, or nonresponders.
Effects of Intramuscular Midazolam and Lorazepam on Acute Agitation in Non-Elderly Subjects - A Systematic Review.[Pubmed:28293921]
Pharmacopsychiatry. 2017 Jul;50(4):129-135.
Benzodiazepines are commonly used for the treatment of acute agitation in a psychiatric setting.We searched MEDLINE, EMBASE, PsycINFO, and the Cochrane Central Register of Controlled Trials (CENTRAL) for relevant publications. Randomized trials evaluating intramuscular (IM) midazolam or Lorazepam given as monotherapy or as add-on treatment, with more than 10 patients aged 18-65 years, conducted in a psychiatric setting, and published between January 1, 1980, and February 3, 2016, were included. 16 studies from a search result of 5 516 studies were included. In total, 577 patients were treated with Lorazepam IM 2-4 mg, and 329 patients were treated with midazolam IM 5-15 mg. It is unclear whether Lorazepam IM or midazolam IM is as efficacious as an antipsychotic IM. It is a bit more certain that the combination of benzodiazepines IM and a low dose antipsychotic IM is more efficacious than the benzodiazepine and the antipsychotic alone. However, there is no doubt that benzodiazepines are less likely to be associated with treatment emergent side effects, as compared to antipsychotics.


