HOE 33187Blue fluorescent dyes CAS# 23623-08-7 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 23623-08-7 | SDF | Download SDF |
| PubChem ID | 44386667 | Appearance | Powder |
| Formula | C25H24N6 | M.Wt | 408.5 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | 25℃: DMSO or water Protect from light | ||
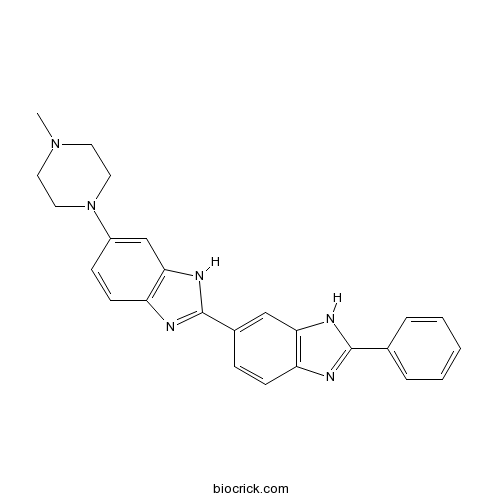
| Chemical Name | 6-(4-methylpiperazin-1-yl)-2-(2-phenyl-3H-benzimidazol-5-yl)-1H-benzimidazole | ||
| SMILES | CN1CCN(CC1)C2=CC3=C(C=C2)N=C(N3)C4=CC5=C(C=C4)N=C(N5)C6=CC=CC=C6 | ||
| Standard InChIKey | SOUKAPYFWOYMNH-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C25H24N6/c1-30-11-13-31(14-12-30)19-8-10-21-23(16-19)29-25(27-21)18-7-9-20-22(15-18)28-24(26-20)17-5-3-2-4-6-17/h2-10,15-16H,11-14H2,1H3,(H,26,28)(H,27,29) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

HOE 33187 Dilution Calculator

HOE 33187 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.448 mL | 12.2399 mL | 24.4798 mL | 48.9596 mL | 61.1995 mL |
| 5 mM | 0.4896 mL | 2.448 mL | 4.896 mL | 9.7919 mL | 12.2399 mL |
| 10 mM | 0.2448 mL | 1.224 mL | 2.448 mL | 4.896 mL | 6.12 mL |
| 50 mM | 0.049 mL | 0.2448 mL | 0.4896 mL | 0.9792 mL | 1.224 mL |
| 100 mM | 0.0245 mL | 0.1224 mL | 0.2448 mL | 0.4896 mL | 0.612 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Description: IC50 Value: N/A Hoechst stains are part of a family of blue fluorescent dyes used to stain DNA. These Bis-benzimides were originally developed by Hoechst AG, which numbered all their compounds so that the dye Hoechst 33342 is the 33342nd compound made by the company. There are three related Hoechst stains: Hoechst 33258, Hoechst 33342, and Hoechst 34580. The dyes Hoechst 33258 and Hoechst 33342 are the ones most commonly used and they have similarexcitation/emission spectra. Both dyes are excited by ultraviolet light at around 350 nm, and both emit blue/cyan fluorescent light around anemission maximum at 461 nm. Unbound dye has its maximum fluorescence emission in the 510-540 nm range. Hoechst dyes are soluble in water and in organic solvents such as dimethyl formamide or dimethyl sulfoxide. Concentrations can be achieved of up to 10 mg/mL. Aqueous solutions are stable at 2-6 °C for at least six months when protected from light. For long-term storage the solutions are instead frozen at ≤-20 °C. The dyes bind to the minor groove of double-stranded DNA with a preference for sequences rich in adenine andthymine. Although the dyes can bind to all nucleic acids, AT-rich double-stranded DNA strands enhance fluorescence considerably. Hoechst dyes are cell-permeable and can bind to DNA in live or fixed cells. Therefore, these stains are often called supravital, which means that cells survive a treatment with these compounds. Cells that express specific ATP-binding cassette transporter proteins can also actively transport these stains out of their cytoplasm. in vitro: N/A in vivo: N/A Clinical trial: N/A
- HOE 32021
Catalog No.:BCC1621
CAS No.:23623-06-5
- [Arg14,Lys15]Nociceptin
Catalog No.:BCC5781
CAS No.:236098-40-1
- H-Phe-pNA
Catalog No.:BCC3010
CAS No.:2360-97-6
- Norisoboldine
Catalog No.:BCN6285
CAS No.:23599-69-1
- Clotrimazole
Catalog No.:BCC3755
CAS No.:23593-75-1
- Procyanidin B3
Catalog No.:BCN6316
CAS No.:23567-23-9
- Hoechst 34580
Catalog No.:BCC1632
CAS No.:23555-00-2
- HOE 32020
Catalog No.:BCC1620
CAS No.:23554-99-6
- Hoechst 33258 analog 3
Catalog No.:BCC1626
CAS No.:23554-98-5
- Daunorubicin HCl
Catalog No.:BCC5083
CAS No.:23541-50-6
- 8-Debenzoylpaeoniflorin
Catalog No.:BCC8787
CAS No.:23532-11-8
- 5-Aza-2'-deoxycytidine
Catalog No.:BCN2169
CAS No.:2353-33-5
- Putraflavone
Catalog No.:BCN5089
CAS No.:23624-21-7
- Kaempferol-3-O-galactoside
Catalog No.:BCN3061
CAS No.:23627-87-4
- Boldenone acetate
Catalog No.:BCC8893
CAS No.:2363-59-9
- Z-Asp(OtBu)-OH.DCHA
Catalog No.:BCC2788
CAS No.:23632-70-4
- Triapine
Catalog No.:BCC5112
CAS No.:236392-56-6
- Clerosterol
Catalog No.:BCN2905
CAS No.:2364-23-0
- Ardisiacrispin A
Catalog No.:BCN2323
CAS No.:23643-61-0
- Neogrifolin
Catalog No.:BCN7526
CAS No.:23665-96-5
- Vicenin -2
Catalog No.:BCN3013
CAS No.:23666-13-9
- Stigmast-4-ene-3,6-dione
Catalog No.:BCN5778
CAS No.:23670-94-2
- Levosulpiride
Catalog No.:BCC4463
CAS No.:23672-07-3
- Boc-Ser(Bzl)-OH
Catalog No.:BCC3442
CAS No.:23680-31-1
Bradykinin Impairs and HOE 140 does not Protect Rat Hindlimb Skeletal Muscle Against Tourniquet-induced Reperfusion Injury.[Pubmed:26375056]
J Invest Surg. 2016;29(1):13-9.
BACKGROUND: Bradykinin (BK) is used in different tissues. Dose-dependent studies have demonstrated that low doses protect against ischemia/reperfusion (I/R) injury while higher doses lead to adverse effects. Although the beneficial effects of BK infusion were observed in myocardium, its role on the I/R impact in skeletal muscle (SM) has not been fully clarified. OBJECTIVE: This study was carried out to evaluate the effects of BK, administered in the hindlimbs of rats subjected to I/R. METHODS: The study design included three experimental groups: Group 1 control (saline), Group 2 (bradykinin), and Group 3 (HOE 140, a BK2 receptor blocker). In all three groups, rats were subjected to hindlimb ischemia for a total of 2 h followed by continuous 4 h of reperfusion with pharmacological interventions. The methods include analysis of enzymes (lactate dehydrogenase-LDH and creatinine phosphokinase-CPK), cell membrane marker of injury (malondialdeyde-MDA), recruitment of neutrophils (myeloperoxidase-MPO), and apoptosis index (immunohistochemistry TUNEL in situ peroxidase dead end). RESULTS: Except for the apoptotic index, all parameters studied were shown to be elevated in the reperfusion group intervened with BK. The blocking of BK2 receptors by HOE 140 did not affect the I/R injury. CONCLUSION: After 2 h of total ischemia, infusion of bradykinin during 4 h of reperfusion, worsened the I/R injury in the hindlimb skeletal muscle.
Aggregate size distribution in a biochar-amended tropical Ultisol under conventional hand-hoe tillage.[Pubmed:28050057]
Soil Tillage Res. 2017 Jan;165:190-197.
Biochar (or pyrogenic organic matter) is increasingly proposed as a soil amendment for improving fertility, carbon sequestration and reduction of greenhouse gas emissions. However, little is known about its effects on aggregation, an important indicator of soil quality and functioning. The aim of this study was to assess the effect of Eucalyptus wood biochar (B, pyrolyzed at 550 degrees C, at 0 or 2.5 t ha(-1)), green manure (T, from Tithonia diversifolia at 0, 2.5 or 5.0 t ha(-1)) and mineral nitrogen (U, urea, at 0, or 120 kg N ha(-1)) on soil respiration, aggregate size distribution and SOC in these aggregate size fractions in a 2-year field experiment on a low-fertility Ultisol in western Kenya under conventional hand-hoe tillage. Air-dry 2-mm sieved soils were divided into four fractions by wet sieving: Large Macro-aggregates (LM; >1000 mum); Small Macro-aggregates (SM, 250-1000 mum); Micro-aggregates (M, 250-53 mum) and Silt + Clay (S + C, < 53 mum). We found that biochar alone did not affect a mean weight diameter (MWD) but combined application with either T. diversifolia (BT) or urea (BU) increased MWD by 34 +/- 5.2 mum (8%) and 55 +/- 5.4 mum (13%), respectively, compared to the control (P = 0.023; n = 36). The B + T + U combination increased the proportion of the LM and SM by 7.0 +/- 0.8%, but reduced the S + C fraction by 5.2 +/- 0.23%. SOC was 30%, 25% and 23% in S + C, M and LM/SM fractions, and increased by 9.6 +/- 1.0, 5.7 +/- 0.8, 6.3 +/- 1.1 and 4.2 +/- 0.9 g kg(-1) for LM, SM, M and S + C, respectively. MWD was not related to either soil respiration or soil moisture but decreased with higher SOC (R(2) = 0.37, P = 0.014, n = 26) and increased with greater biomass production (R(2) = 0.11, P = 0.045, n = 33). Our data suggest that within the timeframe of the study, biochar is stored predominantly as free particulate OC in the silt and clay fraction and promoted a movement of native SOC from larger-size aggregates to the smaller-sized fraction in the short-term (2 years).


