TriapineRibonucleotide reductase inhibitor CAS# 236392-56-6 |
- Gemcitabine HCl
Catalog No.:BCC1076
CAS No.:122111-03-9
- Trovafloxacin mesylate
Catalog No.:BCC3931
CAS No.:147059-75-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 236392-56-6 | SDF | Download SDF |
| PubChem ID | 9571836 | Appearance | Powder |
| Formula | C7H9N5S | M.Wt | 195.24 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in DMSO > 10 mM | ||
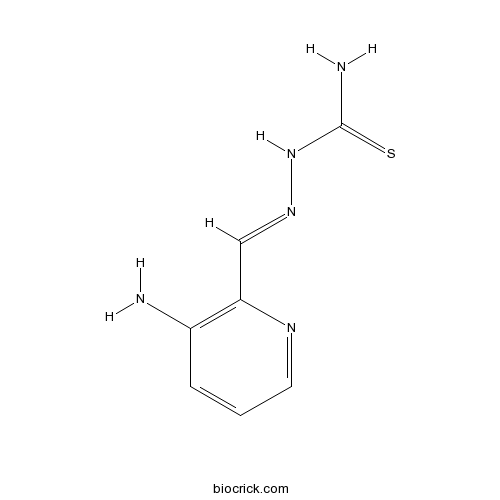
| Chemical Name | [(E)-(3-aminopyridin-2-yl)methylideneamino]thiourea | ||
| SMILES | C1=CC(=C(N=C1)C=NNC(=S)N)N | ||
| Standard InChIKey | XMYKNCNAZKMVQN-NYYWCZLTSA-N | ||
| Standard InChI | InChI=1S/C7H9N5S/c8-5-2-1-3-10-6(5)4-11-12-7(9)13/h1-4H,8H2,(H3,9,12,13)/b11-4+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Triapine is a potent inhibitor of ribonucleotide reductase. | |||||
| Targets | ribonucleotide reductase | |||||
| Cell experiment [1]: | |
| Cell lines | Wild-type (KB) and HU-resistant (KB/HU) human KB nasopharyngeal carcinoma cells. |
| Preparation method | Limited solubility. General tips for obtaining a higher concentration: Please warm the tube at 37 ℃ for 10 minutes and/or shake it in the ultrasonic bath for a while. Stock solution can be stored below -20℃ for several months. |
| Reacting condition | 37oC |
| Applications | Triapine is found to be a much more potent inhibitor of the enzyme than HU regardless of the cellular source of the enzyme, with a comparable inhibition at roughly a 1000-old lower concentration of Triapine than HU. |
| Animal experiment [1]: | |
| Animal models | Mice xenografts of murine M109 lung carcinoma and the human A2780 ovarian carcinoma. |
| Dosage form | i.p. or i.v. bolus injection (0.01 mL/g) |
| Preparation method | Triapine in 0.9% NaCl |
| Application | Triapine significantly inhibits the growth in mice of the M109 lung carcinoma, the twice daily schedule produces tumor growth delays of 10 days compared to untreated control animals. Growth of the human A2780 ovarian carcinoma xenograft in nude mice is also significantly inhibited by Triapine, at 8 and 10 mg/kg given on a twice daily schedule. |
| Other notes | Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
| References: 1. Finch RA, Liu M, Grill SP et al. Triapine (3-aminopyridine-2-carboxaldehyde- thiosemicarbazone): A potent inhibitor of ribonucleotide reductase activity with broad spectrum antitumor activity. Biochem Pharmacol. 2000 Apr 15;59(8):983-91. | |

Triapine Dilution Calculator

Triapine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.1219 mL | 25.6095 mL | 51.219 mL | 102.438 mL | 128.0475 mL |
| 5 mM | 1.0244 mL | 5.1219 mL | 10.2438 mL | 20.4876 mL | 25.6095 mL |
| 10 mM | 0.5122 mL | 2.561 mL | 5.1219 mL | 10.2438 mL | 12.8048 mL |
| 50 mM | 0.1024 mL | 0.5122 mL | 1.0244 mL | 2.0488 mL | 2.561 mL |
| 100 mM | 0.0512 mL | 0.2561 mL | 0.5122 mL | 1.0244 mL | 1.2805 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Triapine is a potent inhibitor of ribonucleotide reductase activity with IC50 value of 1.6 μM for various of tumor cell lines [1].
Triapine has been reported to inhibit ribonucleotide reductase activity. Triapine has shown its antineoplastic activity by inhibiting DNA synthesis and repair. In addition, Triapine has been revealed to inhibit the growth of the murine M109 lung carcinoma and human A2780 ovarian carcinoma xenografts in nude mice. Moreover, Triapine was active against the L1210 leukemia over a broad range of dosages and was curative for the M109 lung carcinoma and human A2780 ovarian carcinoma xenograft mice. [1, 2]
References:
[1]. Jennifer J. Knox, Sebastien J. Hotte, Christian Kollmannsberger, Eric Winquist, Bryn Fisher, Elizabeth A. Eisenhauer .Phase II study of Triapine® in patients with metastatic renal cell carcinoma: a trial of the National Cancer Institute of Canada Clinical Trials Group (NCIC IND.161). nvestigational New Drugs .October 2007, Volume 25, Issue 5, pp 471-477
[2]Finch RA1, Liu M, Grill SP, Rose WC, Loomis R, Vasquez KM, Cheng Y, Sartorelli AC. Triapine (3-aminopyridine-2-carboxaldehyde- thiosemicarbazone): A potent inhibitor of ribonucleotide reductase activity with broad spectrum antitumor activity. Biochem Pharmacol. 2000 Apr 15;59(8):983-91
- Z-Asp(OtBu)-OH.DCHA
Catalog No.:BCC2788
CAS No.:23632-70-4
- Boldenone acetate
Catalog No.:BCC8893
CAS No.:2363-59-9
- Kaempferol-3-O-galactoside
Catalog No.:BCN3061
CAS No.:23627-87-4
- Putraflavone
Catalog No.:BCN5089
CAS No.:23624-21-7
- HOE 33187
Catalog No.:BCC1622
CAS No.:23623-08-7
- HOE 32021
Catalog No.:BCC1621
CAS No.:23623-06-5
- [Arg14,Lys15]Nociceptin
Catalog No.:BCC5781
CAS No.:236098-40-1
- H-Phe-pNA
Catalog No.:BCC3010
CAS No.:2360-97-6
- Norisoboldine
Catalog No.:BCN6285
CAS No.:23599-69-1
- Clotrimazole
Catalog No.:BCC3755
CAS No.:23593-75-1
- Procyanidin B3
Catalog No.:BCN6316
CAS No.:23567-23-9
- Hoechst 34580
Catalog No.:BCC1632
CAS No.:23555-00-2
- Clerosterol
Catalog No.:BCN2905
CAS No.:2364-23-0
- Ardisiacrispin A
Catalog No.:BCN2323
CAS No.:23643-61-0
- Neogrifolin
Catalog No.:BCN7526
CAS No.:23665-96-5
- Vicenin -2
Catalog No.:BCN3013
CAS No.:23666-13-9
- Stigmast-4-ene-3,6-dione
Catalog No.:BCN5778
CAS No.:23670-94-2
- Levosulpiride
Catalog No.:BCC4463
CAS No.:23672-07-3
- Boc-Ser(Bzl)-OH
Catalog No.:BCC3442
CAS No.:23680-31-1
- Olaquindox
Catalog No.:BCN2538
CAS No.:23696-28-8
- Damascenone
Catalog No.:BCN8355
CAS No.:23696-85-7
- Platycoside E
Catalog No.:BCN6385
CAS No.:237068-41-6
- Nardosinone
Catalog No.:BCN2324
CAS No.:23720-80-1
- Chebulic acid
Catalog No.:BCN3260
CAS No.:23725-05-5
Phase I trial of daily triapine in combination with cisplatin chemotherapy for advanced-stage malignancies.[Pubmed:27878356]
Cancer Chemother Pharmacol. 2017 Jan;79(1):201-207.
PURPOSE: Advanced-stage malignancies have increased deoxyribonucleotide demands in DNA replication and repair, making deoxyribonucleotide supply a potential exploitable target for therapy based on ribonucleotide reductase (RNR) inhibition. METHODS: A dose-finding phase I trial was conducted of intravenous (i.v.) Triapine, a small-molecule RNR inhibitor, and cisplatin chemotherapy in patients with advanced-stage solid tumor malignancies. Patients received dose-finding levels of i.v. Triapine (48-96 mg/m(2)) and i.v. cisplatin (20-75 mg/m(2)) on 1 of 3 different schedules. The primary endpoint was to identify the maximum tolerated dose of a Triapine-cisplatin combination. Secondary endpoints included the rate of Triapine-cisplatin objective response and the pharmacokinetics and bioavailability of a single oral Triapine dose. (Clinicaltrials.gov number, NCT00024323). RESULTS: The MTD was 96 mg/m(2) Triapine daily days 1-4 and 75 mg/m(2) cisplatin split over day 2 and day 3. Frequent grade 3 or 4 adverse events included fatigue, dyspnea, leukopenia, thrombocytopenia, and electrolyte abnormalities. No objective responses were observed; 5 (50%) of 10 patients treated at the MTD had stable disease. Pharmacokinetics indicated an oral Triapine bioavailability of 88%. CONCLUSIONS: The Triapine-cisplatin combination may be given safely in patients with advanced-stage solid tumor malignancies. On the basis of these results, a phase I trial adequately powered to evaluate oral Triapine bioavailability in women with advanced-stage uterine cervix or vulvar cancers is underway.
Impact of Stepwise NH2-Methylation of Triapine on the Physicochemical Properties, Anticancer Activity, and Resistance Circumvention.[Pubmed:27336684]
J Med Chem. 2016 Jul 28;59(14):6739-52.
One of the most promising classes of iron chelators are alpha-N-heterocyclic thiosemicarbazones with Triapine as the most prominent representative. In several clinical trials Triapine showed anticancer activity against hematological diseases, however, studies on solid tumors failed due to widely unknown reasons. Some years ago, it was recognized that "terminal dimethylation" of thiosemicarbazones can lead to a more than 100-fold increased activity, probably due to interactions with cellular copper depots. To better understand the structural requirements for the switch to nanomolar cytotoxicity, we systematically synthesized all eight possible N-methylated derivatives of Triapine and investigated their potential against Triapine-sensitive as well as -resistant cell lines. While only the "completely" methylated compound exerted nanomolar activity, the data revealed that all compounds with at least one N-dimethylation were not affected by acquired Triapine resistance. In addition, these compounds were highly synergistic with copper treatment accompanied by induction of reactive oxygen species and massive necrotic cell death.
Triapine potentiates platinum-based combination therapy by disruption of homologous recombination repair.[Pubmed:26964031]
Br J Cancer. 2016 Mar 29;114(7):777-86.
BACKGROUND: Platinum resistance may be attributable to inherent or acquired proficiency in homologous recombination repair (HRR) in epithelial ovarian cancer (EOC). The objective of this study was to evaluate the efficacy of the small molecule inhibitor Triapine to disrupt HRR and sensitise BRCA wild-type EOC cells to platinum-based combination therapy in vitro and in vivo. METHODS: The sensitivity of BRCA wild-type cancer cells to olaparib, cisplatin, carboplatin, doxorubicin, or etoposide in combination with Triapine was evaluated by clonogenic survival assays. The effects of Triapine on HRR activity in cells were measured with a DR-GFP reporter assay. The ability of Triapine to enhance the effects of the carboplatin-doxil combination on EOC tumour growth delay was determined using a xenograft tumour mouse model. RESULTS: Platinum resistance is associated with wild-type BRCA status. Triapine inhibits HRR activity and enhances the sensitivity of BRCA wild-type cancer cells to cisplatin, olaparib, and doxorubicin. However, sequential combination of Triapine and cisplatin is necessary to achieve synergism. Moreover, Triapine potentiates platinum-based combination therapy against BRCA wild-type EOC cells and produces significant delay of EOC tumour growth. CONCLUSIONS: Triapine promises to augment the clinical efficacy of platinum-based combination regimens for treatment of platinum-resistant EOC with wild-type BRCA and proficient HRR activity.
Loss of phosphodiesterase 4D mediates acquired triapine resistance via Epac-Rap1-Integrin signaling.[Pubmed:27602951]
Oncotarget. 2016 Dec 20;7(51):84556-84574.
Triapine, an anticancer thiosemicarbazone, is currently under clinical investigation. Whereas promising results were obtained in hematological diseases, trials in solid tumors widely failed. To understand mechanisms causing Triapine insensitivity, we have analysed genomic alterations in a Triapine-resistant SW480 subline (SW480/tria). Only one distinct genomic loss was observed specifically in SW480/tria cells affecting the phosphodiesterase 4D (PDE4D) gene locus. Accordingly, pharmacological inhibition of PDE4D resulted in significant Triapine resistance in SW480 cells. Hence, we concluded that enhanced cyclic AMP levels might confer protection against Triapine. Indeed, hyperactivation of both major downstream pathways, namely the protein kinase A (PKA)-cAMP response element-binding protein (Creb) and the exchange protein activated by cAMP (Epac)-Ras-related protein 1 (Rap1) signaling axes, was observed in SW480/tria cells. Unexpectedly, inhibition of PKA did not re-sensitize SW480/tria cells against Triapine. In contrast, Epac activation resulted in distinct Triapine resistance in SW480 cells. Conversely, knock-down of Epac expression and pharmacological inhibition of Rap1 re-sensitized SW480/tria cells against Triapine. Rap1 is a well-known regulator of integrins. Accordingly, SW480/tria cells displayed enhanced plasma membrane expression of several integrin subunits, enhanced adhesion especially to RGD-containing matrix components, and bolstered activation/expression of the integrin downstream effectors Src and RhoA/Rac. Accordingly, integrin and Src inhibition resulted in potent Triapine re-sensitization especially of SW480/tria cells. In summary, we describe for the first time integrin activation based on cAMP-Epac-Rap1 signaling as acquired drug resistance mechanism. combinations of Triapine with inhibitors of several steps in this resistance cascade might be feasible strategies to overcome Triapine insensitivity of solid tumors.


