Vicenin -2CAS# 23666-13-9 |
Quality Control & MSDS
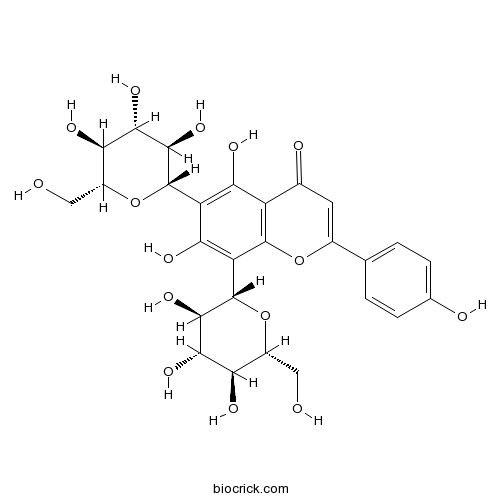
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 23666-13-9 | SDF | Download SDF |
| PubChem ID | 442664 | Appearance | White-yellowish powder |
| Formula | C27H30O15 | M.Wt | 594.5 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Synonyms | 6,8-Di-C-glucosylapigenin; 4',5,7-Trihydroxyflavone 6,8-di-C-glucoside; Violantin | ||
| Solubility | Soluble in ethanol and methanol; sparingly soluble in water | ||
| Chemical Name | 5,7-dihydroxy-2-(4-hydroxyphenyl)-6,8-bis[(2S,3R,4R,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]chromen-4-one | ||
| SMILES | C1=CC(=CC=C1C2=CC(=O)C3=C(O2)C(=C(C(=C3O)C4C(C(C(C(O4)CO)O)O)O)O)C5C(C(C(C(O5)CO)O)O)O)O | ||
| Standard InChIKey | FIAAVMJLAGNUKW-VQVVXJKKSA-N | ||
| Standard InChI | InChI=1S/C27H30O15/c28-6-12-17(32)21(36)23(38)26(41-12)15-19(34)14-10(31)5-11(8-1-3-9(30)4-2-8)40-25(14)16(20(15)35)27-24(39)22(37)18(33)13(7-29)42-27/h1-5,12-13,17-18,21-24,26-30,32-39H,6-7H2/t12-,13-,17-,18-,21+,22+,23-,24-,26+,27+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Vicenin -2 has hepatoprotective, anti-cancer, antioxidant and anti-inflammatory activities, and DTL co-administration is more effective than either of the single agents in androgen-independent prostate cancer. Vicenin -2 might be a useful lead for the development of multiple target-oriented therapeutic modalities for the treatment of diabetes and diabetes-associated complications. Vicenin -2 could act as a UV light barrier to protect the plants. |
| Targets | c-Myc | p53 | EGFR | Akt | mTOR |
| In vitro | Evaluation of intestinal permeability of vicenin-2 and lychnopholic acid from Lychnophora salicifolia (Brazilian arnicão) using Caco-2 cells.[Pubmed: 24279746]J Nat Prod. 2014 Mar 28;77(3):464-71.Lychnophora salicifolia, commonly known as "arnicão", is used as an anti-inflammatory agent and as a flavoring agent in the Brazilian traditional spirit "cachaça". |
| In vivo | Mass spectrometry of flavonoid vicenin-2, based sunlight barriers in Lychnophora species.[Pubmed: 24603617]Sci Rep. 2014 Mar 7;4:4309.Lychnophora salicifolia plants collected from four different places in Brazil (three states: Goias, Minas Gerais and Bahia) revealed a conserved accumulation of Vicenin -2 , a di-C-glycosyl flavonoid. Quantitative studies by UPLC-MS/MS showed high concentration of Vicenin -2 in leaves from sixty specimens of six Lychnophora species. So the tissue distributions of Vicenin -2 were evaluated in wild Lychnophora leaves (Asteraceae) by laser based imaging mass spectrometry (IMS) to propose its distributions and possible functions for the species analyzed. Mass spectrometric imaging revealed that Vicenin -2 , unlike other flavonoids, was produced at the top of the leaves. The combination of localization and UV absorption properties of Vicenin -2 suggests that it could act as a UV light barrier to protect the plants, since plants are sessile organisms that have to protect themselves from harsh external conditions such as intense sunlight. Testing of Perilla frutescens extract and Vicenin 2 for their antispasmodic effect.[Pubmed: 23357362]Phytomedicine. 2013 Mar 15;20(5):427-31.Gastrointestinal discomfort is frequently observed. The effects of Perilla frutescens extract and Vicenin -2 (a compound in this extract) were assayed in rat ileum with or without stimulation with acetylcholine or Ba(2+). Both had no direct spasmolytic effect, but both decreased acetylcholine- or Ba(2+)-induced contraction of rat ileum indicating an antispasmodic effect. This is valuable because effects were only observed when spasms were induced and may disturb the patient. The extract and the compound may be used to maintain and improve gut health. |
| Kinase Assay | Anti-cancer effects of novel flavonoid vicenin-2 as a single agent and in synergistic combination with docetaxel in prostate cancer.[Pubmed: 21803027]Vicenin 2 isolated from Artemisia capillaris exhibited potent anti-glycation properties.[Pubmed: 24713265 ]Food Chem Toxicol. 2014 Jul;69:55-62.Vicenin -2, isolated from a traditionally used medicinal plant Artemisia capillaris, is a 6,8-di-C-glucoside of apigenin which has been previously reported to possess a wide variety of pharmacological activities including antioxidant, anti-inflammatory, anti-cancer, and hepatoprotective. However, there have not been any reports concerning its anti-diabetic potential until now. Biochem Pharmacol. 2011 Nov 1;82(9):1100-9.The present study was conducted to determine the efficacy of novel flavonoid Vicenin -2 (VCN-2), an active constituent of the medicinal herb Ocimum Sanctum Linn or Tulsi, as a single agent and in combination with docetaxel (DTL) in carcinoma of prostate (CaP).
|

Vicenin -2 Dilution Calculator

Vicenin -2 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.6821 mL | 8.4104 mL | 16.8209 mL | 33.6417 mL | 42.0521 mL |
| 5 mM | 0.3364 mL | 1.6821 mL | 3.3642 mL | 6.7283 mL | 8.4104 mL |
| 10 mM | 0.1682 mL | 0.841 mL | 1.6821 mL | 3.3642 mL | 4.2052 mL |
| 50 mM | 0.0336 mL | 0.1682 mL | 0.3364 mL | 0.6728 mL | 0.841 mL |
| 100 mM | 0.0168 mL | 0.0841 mL | 0.1682 mL | 0.3364 mL | 0.4205 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Neogrifolin
Catalog No.:BCN7526
CAS No.:23665-96-5
- Ardisiacrispin A
Catalog No.:BCN2323
CAS No.:23643-61-0
- Clerosterol
Catalog No.:BCN2905
CAS No.:2364-23-0
- Triapine
Catalog No.:BCC5112
CAS No.:236392-56-6
- Z-Asp(OtBu)-OH.DCHA
Catalog No.:BCC2788
CAS No.:23632-70-4
- Boldenone acetate
Catalog No.:BCC8893
CAS No.:2363-59-9
- Kaempferol-3-O-galactoside
Catalog No.:BCN3061
CAS No.:23627-87-4
- Putraflavone
Catalog No.:BCN5089
CAS No.:23624-21-7
- HOE 33187
Catalog No.:BCC1622
CAS No.:23623-08-7
- HOE 32021
Catalog No.:BCC1621
CAS No.:23623-06-5
- [Arg14,Lys15]Nociceptin
Catalog No.:BCC5781
CAS No.:236098-40-1
- H-Phe-pNA
Catalog No.:BCC3010
CAS No.:2360-97-6
- Stigmast-4-ene-3,6-dione
Catalog No.:BCN5778
CAS No.:23670-94-2
- Levosulpiride
Catalog No.:BCC4463
CAS No.:23672-07-3
- Boc-Ser(Bzl)-OH
Catalog No.:BCC3442
CAS No.:23680-31-1
- Olaquindox
Catalog No.:BCN2538
CAS No.:23696-28-8
- Damascenone
Catalog No.:BCN8355
CAS No.:23696-85-7
- Platycoside E
Catalog No.:BCN6385
CAS No.:237068-41-6
- Nardosinone
Catalog No.:BCN2324
CAS No.:23720-80-1
- Chebulic acid
Catalog No.:BCN3260
CAS No.:23725-05-5
- 6-Hydroxy-4-Methylcoumarin
Catalog No.:BCC9206
CAS No.:2373-31-1
- trans-Methylkhellactone
Catalog No.:BCN6919
CAS No.:23733-92-8
- trans-3'-O-Benzoyl-4'-O-methylkhellactone
Catalog No.:BCN6921
CAS No.:23733-95-1
- 4-Amino-3-hydroxybenzoic acid
Catalog No.:BCC8681
CAS No.:2374-03-0
Vicenin 2 isolated from Artemisia capillaris exhibited potent anti-glycation properties.[Pubmed:24713265]
Food Chem Toxicol. 2014 Jul;69:55-62.
Vicenin 2, isolated from a traditionally used medicinal plant Artemisia capillaris, is a 6,8-di-C-glucoside of apigenin which has been previously reported to possess a wide variety of pharmacological activities including antioxidant, anti-inflammatory, anti-cancer, and hepatoprotective. However, there have not been any reports concerning its anti-diabetic potential until now. Therefore, in the present study, we evaluated the anti-diabetic potential of vicenin 2 via alpha-glucosidase, protein tyrosine phosphatase 1B (PTP1B), rat lens aldose reductase (RLAR), and advanced glycation end products (AGE) formation inhibitory assays. Vicenin 2 strongly inhibited alpha-glucosidase, PTP1B, and RLAR in the corresponding assays. In addition, vicenin 2 inhibited the formation of both fluorescent AGE and nonfluorescent AGE, e.g., CML, as well as the level of fructosamine in glucose-fructose-induced bovine serum albumin (BSA) glycation. In the test system, vicenin 2 suppressed glycation-induced protein oxidation by attenuating the formation of protein carbonyl groups as well as by inhibiting the modification of protein thiol groups. Moreover, vicenin 2 was found to be a potent inhibitor of glycation-induced formation of amyloid cross-beta structures in BSA. Taken together, vicenin 2 might be a useful lead for the development of multiple target-oriented therapeutic modalities for the treatment of diabetes and diabetes-associated complications.
Testing of Perilla frutescens extract and Vicenin 2 for their antispasmodic effect.[Pubmed:23357362]
Phytomedicine. 2013 Mar 15;20(5):427-31.
Gastrointestinal discomfort is frequently observed. The effects of Perilla frutescens extract and Vicenin 2 (a compound in this extract) were assayed in rat ileum with or without stimulation with acetylcholine or Ba(2+). Both had no direct spasmolytic effect, but both decreased acetylcholine- or Ba(2+)-induced contraction of rat ileum indicating an antispasmodic effect. This is valuable because effects were only observed when spasms were induced and may disturb the patient. The extract and the compound may be used to maintain and improve gut health.
Anti-cancer effects of novel flavonoid vicenin-2 as a single agent and in synergistic combination with docetaxel in prostate cancer.[Pubmed:21803027]
Biochem Pharmacol. 2011 Nov 1;82(9):1100-9.
The present study was conducted to determine the efficacy of novel flavonoid vicenin-2 (VCN-2), an active constituent of the medicinal herb Ocimum Sanctum Linn or Tulsi, as a single agent and in combination with docetaxel (DTL) in carcinoma of prostate (CaP). VCN-2 effectively induced anti-proliferative, anti-angiogenic and pro-apoptotic effect in CaP cells (PC-3, DU-145 and LNCaP) irrespective of their androgen responsiveness or p53 status. VCN-2 inhibited EGFR/Akt/mTOR/p70S6K pathway along with decreasing c-Myc, cyclin D1, cyclin B1, CDK4, PCNA and hTERT in vitro. VCN-2 reached a level of 2.6+/-0.3mumol/l in serum after oral administration in mice which reflected that VCN-2 is orally absorbed. The i.v. administration of docetaxel (DTL), current drug of choice in androgen-independent CaP, is associated with dose-limiting toxicities like febrile neutropenia which has lead to characterization of alternate routes of administration and potential combinatorial regimens. In this regard, VCN-2 in combination with DTL synergistically inhibited the growth of prostate tumors in vivo with a greater decrease in the levels of AR, pIGF1R, pAkt, PCNA, cyclin D1, Ki67, CD31, and increase in E-cadherin. VCN-2 has been investigated for radioprotection and anti-inflammatory properties. This is the first study on the anti-cancer effects of VCN-2. In conclusion, our investigations collectively provide strong evidence that VCN-2 is effective against CaP progression along with indicating that VCN-2 and DTL co-administration is more effective than either of the single agents in androgen-independent prostate cancer.
Evaluation of intestinal permeability of vicenin-2 and lychnopholic acid from Lychnophora salicifolia (Brazilian arnicao) using Caco-2 cells.[Pubmed:24279746]
J Nat Prod. 2014 Mar 28;77(3):464-71.
Lychnophora salicifolia, commonly known as "arnicao", is used as an anti-inflammatory agent and as a flavoring agent in the Brazilian traditional spirit "cachaca". In this work, the permeation process of vicenin-2 (1) and lychnopholic acid (2) (major secondary metabolites from the hydroalcoholic extract) was investigated using Caco-2 cells. For this investigation, a new HPLC-DAD method was developed and validated for the quantification step. It was observed that 2 crosses the Caco-2 cell monolayer by passive diffusion. On the other hand, 1 was not transported, suggesting no absorption and no efflux of this compound in Caco-2 cells.
Mass spectrometry of flavonoid vicenin-2, based sunlight barriers in Lychnophora species.[Pubmed:24603617]
Sci Rep. 2014 Mar 7;4:4309.
Lychnophora salicifolia plants collected from four different places in Brazil (three states: Goias, Minas Gerais and Bahia) revealed a conserved accumulation of vicenin-2, a di-C-glycosyl flavonoid. Quantitative studies by UPLC-MS/MS showed high concentration of vicenin-2 in leaves from sixty specimens of six Lychnophora species. So the tissue distributions of vicenin-2 were evaluated in wild Lychnophora leaves (Asteraceae) by laser based imaging mass spectrometry (IMS) to propose its distributions and possible functions for the species analyzed. Mass spectrometric imaging revealed that vicenin-2, unlike other flavonoids, was produced at the top of the leaves. The combination of localization and UV absorption properties of vicenin-2 suggests that it could act as a UV light barrier to protect the plants, since plants are sessile organisms that have to protect themselves from harsh external conditions such as intense sunlight.


