LevosulpirideAntipsychotic drug CAS# 23672-07-3 |
- Leucovorin Calcium
Catalog No.:BCC1198
CAS No.:6035-45-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 23672-07-3 | SDF | Download SDF |
| PubChem ID | 5355 | Appearance | Powder |
| Formula | C15H23N3O4S | M.Wt | 341.43 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 50 mg/mL (146.44 mM; Need ultrasonic) | ||
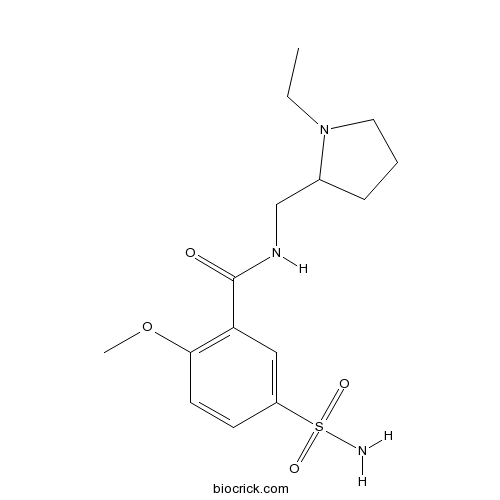
| Chemical Name | N-[(1-ethylpyrrolidin-2-yl)methyl]-2-methoxy-5-sulfamoylbenzamide | ||
| SMILES | CCN1CCCC1CNC(=O)C2=C(C=CC(=C2)S(=O)(=O)N)OC | ||
| Standard InChIKey | BGRJTUBHPOOWDU-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C15H23N3O4S/c1-3-18-8-4-5-11(18)10-17-15(19)13-9-12(23(16,20)21)6-7-14(13)22-2/h6-7,9,11H,3-5,8,10H2,1-2H3,(H,17,19)(H2,16,20,21) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Active enantiomer. Selective D2-like dopamine antagonist (Ki values are ~ 0.015. ~ 0.013, 1, ~ 45 and ~ 77 μM at D2, D3, D4, D1 and D5 receptors respectively). |

Levosulpiride Dilution Calculator

Levosulpiride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.9289 mL | 14.6443 mL | 29.2886 mL | 58.5772 mL | 73.2215 mL |
| 5 mM | 0.5858 mL | 2.9289 mL | 5.8577 mL | 11.7154 mL | 14.6443 mL |
| 10 mM | 0.2929 mL | 1.4644 mL | 2.9289 mL | 5.8577 mL | 7.3221 mL |
| 50 mM | 0.0586 mL | 0.2929 mL | 0.5858 mL | 1.1715 mL | 1.4644 mL |
| 100 mM | 0.0293 mL | 0.1464 mL | 0.2929 mL | 0.5858 mL | 0.7322 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Levosulpiride is a substituted benzamide antipsychotic, reported to be a selective antagonist of dopamine D2 receptor activity on both central and peripheral levels. It is an atypical neuroleptic and a prokinetic agent. Levosulpiride is also claimed to ha
- Stigmast-4-ene-3,6-dione
Catalog No.:BCN5778
CAS No.:23670-94-2
- Vicenin -2
Catalog No.:BCN3013
CAS No.:23666-13-9
- Neogrifolin
Catalog No.:BCN7526
CAS No.:23665-96-5
- Ardisiacrispin A
Catalog No.:BCN2323
CAS No.:23643-61-0
- Clerosterol
Catalog No.:BCN2905
CAS No.:2364-23-0
- Triapine
Catalog No.:BCC5112
CAS No.:236392-56-6
- Z-Asp(OtBu)-OH.DCHA
Catalog No.:BCC2788
CAS No.:23632-70-4
- Boldenone acetate
Catalog No.:BCC8893
CAS No.:2363-59-9
- Kaempferol-3-O-galactoside
Catalog No.:BCN3061
CAS No.:23627-87-4
- Putraflavone
Catalog No.:BCN5089
CAS No.:23624-21-7
- HOE 33187
Catalog No.:BCC1622
CAS No.:23623-08-7
- HOE 32021
Catalog No.:BCC1621
CAS No.:23623-06-5
- Boc-Ser(Bzl)-OH
Catalog No.:BCC3442
CAS No.:23680-31-1
- Olaquindox
Catalog No.:BCN2538
CAS No.:23696-28-8
- Damascenone
Catalog No.:BCN8355
CAS No.:23696-85-7
- Platycoside E
Catalog No.:BCN6385
CAS No.:237068-41-6
- Nardosinone
Catalog No.:BCN2324
CAS No.:23720-80-1
- Chebulic acid
Catalog No.:BCN3260
CAS No.:23725-05-5
- 6-Hydroxy-4-Methylcoumarin
Catalog No.:BCC9206
CAS No.:2373-31-1
- trans-Methylkhellactone
Catalog No.:BCN6919
CAS No.:23733-92-8
- trans-3'-O-Benzoyl-4'-O-methylkhellactone
Catalog No.:BCN6921
CAS No.:23733-95-1
- 4-Amino-3-hydroxybenzoic acid
Catalog No.:BCC8681
CAS No.:2374-03-0
- Rivulobirin E
Catalog No.:BCN5090
CAS No.:237407-59-9
- Methylprednisolone Sodium Succinate
Catalog No.:BCC5629
CAS No.:2375-03-3
A Comparative Study of Trigger Point Therapy with Local Anaesthetic (0.5 % Bupivacaine) Versus Combined Trigger Point Injection Therapy and Levosulpiride in the Management of Myofascial Pain Syndrome in the Orofacial Region.[Pubmed:27752210]
J Maxillofac Oral Surg. 2016 Sep;15(3):376-383.
AIM: To compare the efficacy of combined local anesthetic injection with 0.5 % bupivacaine and Levosulpiride versus local anesthetic injection alone on outcome measures including levels of pain intensity and depression in the management of myofascial pain syndrome in orofacial region. PATIENTS AND METHODS: This was a prospective, randomized, controlled and open-label comparative clinical study. Seventy-four patients diagnosed to have myofascial pain syndrome and fulfilling the inclusion criteria were enrolled for the study. Patients were randomly assigned into 2 groups. Group A received local anesthetic injection (0.5 % bupivacaine) on trigger points and Group B received combined trigger point injection therapy and 50 mg of tablet Levosulpiride orally B.I.D. They were assessed for pain intensity and depression at baseline and at follow-up of 1, 4, 6 and 12 week intervals. RESULTS: The mean age of patients was 44.54 + 15.977 years in group A and 39.97 + 14.107 years in group B (P value = 0.2). Group A comprised of 25 females (67.567 %) and 12 males (32.432 %) while group B had 27 females (75 %) and 9 males (25 %). 70.27 % were diagnosed with moderate depression in group A and 75 % in group B. 18.91 % in group A and 19.44 % in group B were diagnosed with severe depression. When the VAS score and BDI score was compared at the follow-up intervals with the baseline scores in both treatment groups, the mean difference was highly significant at all the follow-up intervals. However when the relative efficacies of two interventions were compared between the two groups, improvement in pain was significant at all the follow-up intervals except the 1st week follow-up whereas the improvement in depression was non-significant at 1st and 4th week interval while it was highly significant at 6th and 12th week intervals. CONCLUSION: The combined therapy with trigger point injection and Levosulpiride as antidepressant significantly reduces pain and depression in the study subjects suffering from chronic myofascial pain with moderate to severe depression in the orofacial region.
Formulation, characterization, in vitro and in vivo evaluation of castor oil based self-nano emulsifying levosulpiride delivery systems.[Pubmed:27599558]
J Microencapsul. 2016 Sep;33(6):535-543.
CONTEXT: Levosulpiride (LSP) is a hydrophobic benzamide derivative used in the treatment of schizophrenia. SNEDDS were extensively practiced for systemic delivery of poorly aqueous soluble drugs to achieve maximum bioavailability. OBJECTIVE: The present study was focussed on the formulation, optimisation and evaluation of LSP SNEDDS using castor oil, for enhancement of drug absorption and bioavailability. MATERIALS AND METHODS: Pseudo-ternary phase diagram was plotted to identify the range of SNEDDS components. Twenty formulations were designed, prepared and characterised by its particle size, zeta potential, viscosity, and stability. In vitro dissolution data modelling was performed. Microscopy, FTIR and in vivo bioavailability studies were conducted for optimum formulation. Results, discussion and conclusion: F18 containing castor oil, 0.9 mL; PEG 600, 1.36 mL and Tween 80, 2.74 mL was found to be optimum. The optimised formulation had shown uniform globule size, no interactions of LSP with SNEDDS components and higher pharmacokinetic parameters than that of commercial preparation.
Bioequivalence of a New Oral Levosulpiride Formulation Compared With a Standard One in Healthy Volunteers.[Pubmed:28196046]
Ther Drug Monit. 2017 Apr;39(2):118-123.
BACKGROUND: A monocentric, single-dose, open-label, 2-way, crossover randomized study was conducted by the San Matteo Phase I Clinical Trial Unit and Experimental Therapy (Pavia, Italy) to assess the bioequivalence and the systemic tolerability of a new oral formulation of Levosulpiride (tablet 25 mg: test) versus a commercially available formulation on the Italian market (tablet 25 mg: reference). METHODS: Thirty-five healthy adult volunteers, men (n = 19) and women (n = 16), aged between 18 and 55 years were screened and 32 of them were enrolled in the study. After having signed the written informed consent, each subject received a single oral dose of Test or Reference product with 250 mL of natural mineral water, in fasting conditions, interspersed with a 6-day washout period Blood samples were collected up to 36 hours after drug administration: the drug plasma levels were determined by high-performance liquid chromatography coupled with electrospray ionization tandem mass spectrometry. The pharmacokinetic parameters included peak plasma concentration (Cmax), time corresponding to Cmax (tmax), area under the plasma concentration-time curve from zero to infinity (AUC0-infinity) or to the last sampling time assessment (AUC0-36), the elimination rate constant (ke), and the terminal half-life (t1/2). Safety was measured by pre- and post-treatment specific biochemical investigations, physical examination, electrocardiogram, occurrence of adverse events, and any information on patients' withdrawal. RESULTS: The geometric mean ratio Test/Reference (90% confidence interval) for Levosulpiride was 103.0% (95.8-110.8) for AUC0-36, 103.6% (95.9-111.9) for AUC0-infinity, and 104.3% (94.9-114.6) for Cmax. ke and t1/2 were 0.07 (SD: 0.02) and 9 hours (8-12) for both the formulations. Clearance (L/h) was 29.6 (+/-13.5) and 30.7 (+/-14.2) for the test and the reference product, respectively. CONCLUSIONS: Because the acceptance criteria required by the drug regulatory agency (European Medicines Agency, EMA) for bioequivalence prescribe limits of 80%-120% for untransformed data and 80%-125% for "ln" transformed data, we can confirm that the 2 formulations are bioequivalent, in terms of the rate and extent of absorption.


