HorminoneCAS# 21887-01-4 |
- Taxoquinone
Catalog No.:BCN6660
CAS No.:21764-41-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 21887-01-4 | SDF | Download SDF |
| PubChem ID | 2751795 | Appearance | Yellow powder |
| Formula | C20H28O4 | M.Wt | 332.4 |
| Type of Compound | Diterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
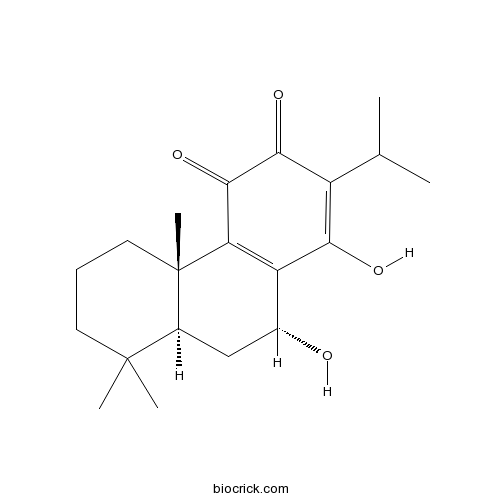
| Chemical Name | (4bS,8aS,10R)-1,10-dihydroxy-4b,8,8-trimethyl-2-propan-2-yl-5,6,7,8a,9,10-hexahydrophenanthrene-3,4-dione | ||
| SMILES | CC(C)C1=C(C2=C(C(=O)C1=O)C3(CCCC(C3CC2O)(C)C)C)O | ||
| Standard InChIKey | WAZYPYJGZYLHHT-JGRMJRGVSA-N | ||
| Standard InChI | InChI=1S/C20H28O4/c1-10(2)13-16(22)14-11(21)9-12-19(3,4)7-6-8-20(12,5)15(14)18(24)17(13)23/h10-12,21-22H,6-9H2,1-5H3/t11-,12+,20+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Horminone may inhibit the initial steps of protein synthesis. 2. After administration of plant extracts containing Horminone has possibility of toxic effect. 3. Horminone and tingenone can inhibit the in vitro growth of Trypanosoma cruzi, 30 microM drug concentration producing total inhibition of growth. 4. Horminone has antimicrobial activity, it can inhibit the protein synthesis in several types of bacteria. 5. Horminone displays marked concentration-dependent antiproliferative effects. |
| Targets | DNA/RNA Synthesis | NADPH-oxidase | Antifection |

Horminone Dilution Calculator

Horminone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.0084 mL | 15.0421 mL | 30.0842 mL | 60.1685 mL | 75.2106 mL |
| 5 mM | 0.6017 mL | 3.0084 mL | 6.0168 mL | 12.0337 mL | 15.0421 mL |
| 10 mM | 0.3008 mL | 1.5042 mL | 3.0084 mL | 6.0168 mL | 7.5211 mL |
| 50 mM | 0.0602 mL | 0.3008 mL | 0.6017 mL | 1.2034 mL | 1.5042 mL |
| 100 mM | 0.0301 mL | 0.1504 mL | 0.3008 mL | 0.6017 mL | 0.7521 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Lycorine chloride
Catalog No.:BCN1220
CAS No.:2188-68-3
- Boc-Arg(NO2)-OH
Catalog No.:BCC3065
CAS No.:2188-18-3
- 12-Hydroxyisodrimenin
Catalog No.:BCN4935
CAS No.:218780-16-6
- Boc- ß-HoIle-OH
Catalog No.:BCC3236
CAS No.:218608-82-3
- Bardoxolone methyl
Catalog No.:BCC1400
CAS No.:218600-53-4
- Bardoxolone
Catalog No.:BCC1399
CAS No.:218600-44-3
- Falcarinol
Catalog No.:BCN3938
CAS No.:21852-80-2
- Octahydroisoindole
Catalog No.:BCN2275
CAS No.:21850-12-4
- BDNF (human)
Catalog No.:BCC5944
CAS No.:218441-99-7
- Macrocarpal K
Catalog No.:BCN4934
CAS No.:218290-59-6
- Nifedipine
Catalog No.:BCC4808
CAS No.:21829-25-4
- Vindoline
Catalog No.:BCN4933
CAS No.:2182-14-1
- Boc-Orn-OH
Catalog No.:BCC3427
CAS No.:21887-64-9
- 1-Phenyloctane
Catalog No.:BCN2227
CAS No.:2189-60-8
- Taraxeryl acetate
Catalog No.:BCN4937
CAS No.:2189-80-2
- Ciwujiatone
Catalog No.:BCN7598
CAS No.:218901-26-9
- Euphorbia factor L2
Catalog No.:BCN3783
CAS No.:218916-51-9
- 5,15-Diacetyl-3-benzoyllathyrol
Catalog No.:BCN1196
CAS No.:218916-52-0
- Euphorbia factor L8
Catalog No.:BCN3785
CAS No.:218916-53-1
- KNK437
Catalog No.:BCC6399
CAS No.:218924-25-5
- Z-Trp(Boc)-OH.DCHA
Catalog No.:BCC2749
CAS No.:218938-57-9
- Boc-ß-HoGlu(OBzl)-OH
Catalog No.:BCC3233
CAS No.:218943-30-7
- 2,3-Dihydro-3-methoxywithaferin A
Catalog No.:BCN7943
CAS No.:21902-96-5
- 3'-Methoxydaidzein
Catalog No.:BCN4082
CAS No.:21913-98-4
Effect of tingenone, a quinonoid triterpene, on growth and macromolecule biosynthesis in Trypanosoma cruzi.[Pubmed:3888660]
Experientia. 1985 May 15;41(5):646-8.
Tingenone and Horminone, two natural quinonoid substances, inhibited the in vitro growth of Trypanosoma cruzi, 30 microM drug concentration producing total inhibition of growth. Tingenone inhibited total uptake and incorporation of [3H]thymidine, [3H]uridine, L-[3H]leucine into parasite macromolecules. Other quinonoids assayed were either less effective (abruquinone A) or even quite inactive (visminone B and ferruginin B). Investigation of several mechanisms for the cytotoxic action of tingenone pointed to the interaction with DNA as the most likely factor involved. Tingenone also inhibited the growth of Crithidia fasciculata, but the drug was significantly less active on this organism than on T. cruzi.
Effects of horminone on liver mixed function mono-oxygenases and glutathione enzyme activities of Wistar rat.[Pubmed:9324001]
J Ethnopharmacol. 1997 Sep;58(1):21-30.
The present study reports on the effects of Horminone on serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels, on hepatic cytochrome P450 (P450) and cytochrome b5 (cyt b5) contents and on the activities of NADPH-cytochrome P450 reductase (NR), mixed function mono-oxygenases (MFO), glutathione-S-transferase (GST) and glutathione reductase (GR) of Wistar male rat. Horminone is a diterpenoid quinone (7,12-dihydroxyabiet-8,12-diene-11,14-dione) present in several species of the Labiatae family and used as medicinal plants in folk medicine. In this study, Horminone was administered by the intraperitoneal route (i.p.) at a concentration of 1 or 10 mg/kg to each group of six mice, using water as a vehicle. On the one hand, results showed that Horminone increased serum ALT and AST levels and cyt b5 content and induced the activities of ethylmorphine N-demethylase (EMD). On the other hand, Horminone decreased P450 content and inhibited the activities of 7-ethoxyresorufin O-deethylase (ERD), 7-ethoxycoumarin O-deethylase (ECD), aniline 4-hydroxylase (AH) and NR. Based on these results, the possibility of toxic effects occurring after administration of plant extracts containing Horminone must be considered.
Bioactivity-guided study of antiproliferative activities of Salvia extracts.[Pubmed:21615011]
Nat Prod Commun. 2011 May;6(5):575-9.
The cytotoxic activities of the n-hexane, chloroform and aqueous methanolic fractions prepared from the methanolic extract of the leaves of 23 Salvia taxa were studied for their cell growth-inhibitory activity against human cervix adenocarcinoma (HeLa), skin carcinoma (A431) and breast adenocarcinoma (MCF7) cells using the MTT assay. The n-hexane fractions of six Salvia taxa (S. hispanica, S. nemorosa, S. nemorosa 1. albiflora, S. pratensis, S. recognita and S. ringens) and the chloroform fraction ofS. officinalis 1. albiflora produced over 50% growth inhibition of the skin carcinoma cell line. None of the tested extracts showed substantial (above 50%) antiproliferative effects against HeLa and MCF7 cells. S. ringens was the most powerful among the studied Salvia species with a 61.8% cell growth inhibitory activity on A431 cells. In the case of S. ringens, other plant parts were also tested for antiproliferative effect, and the highest activities were recorded for the root extract. This was subjected to bioactivity-guided fractionation, which yielded four abietane diterpenes (royleanone, Horminone, 7-O-methyl-Horminone and 7-acetyl-Horminone), one triterpene (erythrodiol-3-acetate) and beta-sitosterol. Horminone, 7-acetyl-Horminone and erythrodiol-3-acetate displayed marked concentration-dependent antiproliferative effects, while royleanone and 7-O-methyl-Horminone produced weaker activities.
Theoretical study of the complexes of horminone with Mg2+ and Ca2+ ions and their relation with the bacteriostatic activity.[Pubmed:16571064]
J Phys Chem A. 2006 Apr 6;110(13):4564-73.
The coordination of the Horminone molecule with hydrated magnesium and calcium divalent ions was studied by means of the density functional theory. All-electron calculations were performed with the B3LYP/6-31G method. The first layer of the water molecules surrounding the metallic cations was included. It was found that the octahedral [Horminone(O(a)-O(d))-Mg-(H(2)O)(4)](2+) complex is more stable than [Mg(H(2)O)(6)](2+). That is, Horminone is able to displace two water units from the hexahydrated complex. This behavior does not occur for Ca(2+). Consistently, [Horminone(O(a)-O(d))-Mg-(H(2)O)(4)](2+) has a greater metal-ligand binding energy than [Horminone(O(a)-O(d))-Ca-(H(2)O)(4)](2+). The preference of Horminone by Mg(2+) is enlightened by these results. Moreover, its electronic structure, as shown by huge changes in the atomic populations, is strongly perturbed by Mg(2+). Indeed, Horminone, bonded to [Mg(H(2)O)(4)](2+), is able to cross the bacterial membrane cell. Once inside, [Horminone(O(a)-O(d))-Mg-(H(2)O)(4)](2+) binds to rRNA phosphate groups yielding [Horminone(O(a)-O(d))-Mg-(H(2)O)(PO(4)H(2))(PO(4)H(3))(2)](+). These results give insights into how Horminone may inhibit the initial steps of protein synthesis. The stability of the studied systems is accounted for in terms of the calculated structural and electronic properties: Mg-O and Ca-O bond lengths, charge transfers, and binding energies.


