Hydroxypinacolone RetinoateCAS# 893412-73-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 893412-73-2 | SDF | Download SDF |
| PubChem ID | 25054592.0 | Appearance | Powder |
| Formula | C26H38O3 | M.Wt | 398.58 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
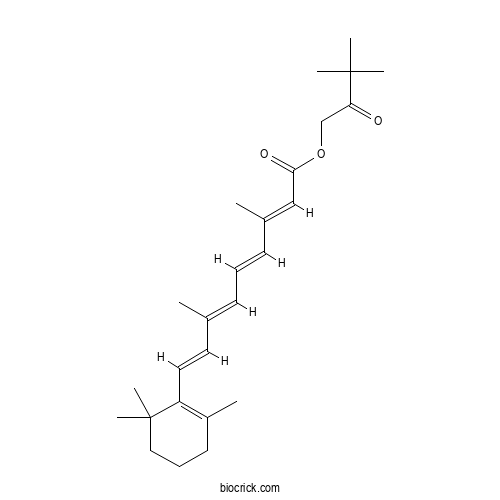
| Chemical Name | (3,3-dimethyl-2-oxobutyl) (2E,4E,6E,8E)-3,7-dimethyl-9-(2,6,6-trimethylcyclohexen-1-yl)nona-2,4,6,8-tetraenoate | ||
| SMILES | CC1=C(C(CCC1)(C)C)C=CC(=CC=CC(=CC(=O)OCC(=O)C(C)(C)C)C)C | ||
| Standard InChIKey | XLPLFRLIWKRQFT-XUJYDZMUSA-N | ||
| Standard InChI | InChI=1S/C26H38O3/c1-19(14-15-22-21(3)13-10-16-26(22,7)8)11-9-12-20(2)17-24(28)29-18-23(27)25(4,5)6/h9,11-12,14-15,17H,10,13,16,18H2,1-8H3/b12-9+,15-14+,19-11+,20-17+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Hydroxypinacolone Retinoate Dilution Calculator

Hydroxypinacolone Retinoate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.5089 mL | 12.5445 mL | 25.0891 mL | 50.1781 mL | 62.7227 mL |
| 5 mM | 0.5018 mL | 2.5089 mL | 5.0178 mL | 10.0356 mL | 12.5445 mL |
| 10 mM | 0.2509 mL | 1.2545 mL | 2.5089 mL | 5.0178 mL | 6.2723 mL |
| 50 mM | 0.0502 mL | 0.2509 mL | 0.5018 mL | 1.0036 mL | 1.2545 mL |
| 100 mM | 0.0251 mL | 0.1254 mL | 0.2509 mL | 0.5018 mL | 0.6272 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 2-Methoxybenzoicacid
Catalog No.:BCX0933
CAS No.:529-75-9
- Nicotinamidemononucleotide
Catalog No.:BCX0932
CAS No.:1094-61-7
- Cepharanoline
Catalog No.:BCX0931
CAS No.:27686-34-6
- PhysalinF
Catalog No.:BCX0930
CAS No.:57517-46-1
- 3-(3-hydroxylphenyl)propanol
Catalog No.:BCX0929
CAS No.:621-54-5
- (+)-Pinoresinolmonomethylether4-O-β-D-glucoside
Catalog No.:BCX0928
CAS No.:74957-57-6
- Hydroxyisogermafurenolide
Catalog No.:BCX0927
CAS No.:20267-91-8
- 14-Deoxy-11-oxoandrographolide
Catalog No.:BCX0926
CAS No.:42895-57-8
- MogrosideIIA
Catalog No.:BCX0925
CAS No.:1613527-65-3
- Pseudojervine
Catalog No.:BCX0924
CAS No.:36069-05-3
- BoeravinoneA
Catalog No.:BCX0923
CAS No.:114567-33-8
- D-Pyroglutamicacid
Catalog No.:BCX0922
CAS No.:4042-36-8
- Coumarin 343
Catalog No.:BCX0935
CAS No.:55804-65-4
- LINOLELAIDICACIDMETHYLESTER
Catalog No.:BCX0936
CAS No.:2566-97-4
- Ginno
Catalog No.:BCX0937
CAS No.:2606-50-0
- β-Elemene
Catalog No.:BCX0938
CAS No.:515-13-9
- PhlorigidosideC
Catalog No.:BCX0939
CAS No.:276691-32-8
- (S)-(-)-Norcoclaurinehydrobromide
Catalog No.:BCX0940
CAS No.:105990-27-0
- 14-hydroxylatedbrassinosteroid
Catalog No.:BCX0941
CAS No.:457603-63-3
- Harmalinehydrochloridedihydrate
Catalog No.:BCX0942
CAS No.:6027-98-1
- Lycobetaineacetate
Catalog No.:BCX0943
CAS No.:61221-41-8
- CompoundK
Catalog No.:BCX0944
CAS No.:160729-91-9
- 11-hydroxy-1-isomangostin
Catalog No.:BCX0945
CAS No.:164365-71-3
- GarcixanthonesB
Catalog No.:BCX0946
CAS No.:2522597-99-3
Evaluation of the safety and efficacy of cosmetics ingredient Spherulites Paeony Superior Retinol.[Pubmed:38665039]
J Cosmet Dermatol. 2024 Apr 25.
BACKGROUND: Microencapsulation of Hydroxypinacolone Retinoate (HPR) can improve its application in cosmetics. OBJECTIVE: To investigate the safety and efficacy of Spherulites Paeony Superior Retinol, a HPR microcapsule containing 5%-10% peony seed oil, 0.01%-1% epigallocatechin gallatyl glucoside (ECGG), and 0.1%-1% HPR. METHODS: The safety of Spherulites Paenoy Superior Retinol was evaluated with zebrafish embryo self-rotation irritation test and developmental toxicity test. SymRenew HPR was used as a reference. The skin care efficacies of Spherulites Paenoy Superior Retinol were evaluated using zebrafish embryos covering antioxidation, anti-inflammation, blood circulation, whitening, wound healing, skin barrier protection, Type I collagen, elastin, and 5alpha-reductase genes expression activities. RESULTS: The irritation test revealed that 250 mug/mL Spherulites Paenoy Superior Retinol did not, while 20 mug/mL SymRenew HPR significantly (p < 0.05) increased zebrafish embryo self-rotation frequency. The developmental toxicity test found the teratogenicity index (half lethal concentration/half toxicity concentration) of Spherulites Paenoy Superior Retinol and SymRenew HPR were 1.9 and 3.1, respectively. The efficacy analysis results showed that 5 mug/mL Spherulites Paenoy Superior Retinol significantly (p < 0.05) exerted 7.1% anti-ROS, 20% anti-inflammation, 14% enhanced blood circulation, 10% suppressed melanin synthesis, 9% enhanced tail fin regeneration, 72% elicited skin barrier protection activity, enhanced the expression of Type I collagen genes col1a1, col1a2, and col1a2 by 34%, 51%, and 42%, respectively, and elastin gene elna by 46%, and suppressed the expression of 5alpha-reductase genes srd5a1, srd5a2a, and srd5a2b by 52%, 15%, and 30%, respectively. CONCLUSION: This study demonstrated that Spherulites Paenoy Superior Retinol is a safe cosmetic ingredient with multi-skin care efficacies.
Comparison of five retinoids for anti-photoaging therapy: Evaluation of anti-inflammatory and anti-oxidative activities in vitro and therapeutic efficacy in vivo.[Pubmed:37990342]
Photochem Photobiol. 2023 Nov 21.
Over the past decades, increasing evidences have demonstrated that five retinoids, including retinol (ROL), retinol acetate (RAc), retinol propionate (RP), retinol palmitate (RPalm), and Hydroxypinacolone Retinoate (HPR), can be potential therapeutic agents for skin photoaging. However, therapeutic efficacies and biosafety have never been compared to these compounds. This study aimed to determine the optimal retinoid type(s) for anti-photoaging therapy both in vitro and in vivo. Our data demonstrated that four retinoids (RPalm, RP, HPR and ROL) but not RAc were effective for anti-photoaging treatment at 5 mug/mL in vitro, with action mechanisms associated with antioxidative, anti-inflammatory and anti-skin ECM degradation activities. Notably, both RPalm and RP appeared superior to HPR and ROL for those activities. Importantly, both RPalm and RP were shown to be optimal for anti-photoaging therapy when topically applied at 5 mg/kg in a UVB-induced mice model of photoaging, which is consistent with their high anti-photoaging activities in vitro. Additionally, topical application of these five retinoids showed satisfactory biosafety without causing significant apoptosis in animal organs, although RP application led to a slight decline in animal body weights. Collectively, these data have laid a good foundation for the next development of the clinical application of these retinoids for skin healthcare.
Characterization of Acne-Prone Skin with Reflectance Confocal Microscopy and Optical Coherence Tomography and Modifications Induced by Topical Treatment and Probiotic Supplementation.[Pubmed:37510902]
J Clin Med. 2023 Jul 20;12(14):4787.
The evaluation of acne-prone skin and absent-to-mild acne is difficult because this condition is not associated with a clinically definable situation. Previous studies showed that apparently healthy skin in patients with previous episodes of acne shows microcomedos and infundibular hyperkeratosis upon reflectance confocal microscopy (RCM) evaluation. Our aim was to characterize the subclinical and microscopic characteristics of acne-prone skin by means of RCM and dynamic optical coherence tomography (D-OCT) and evaluate microscopic changes induced by treatment. A group of 20 patients received a daily combined treatment over a period of 3 months, consisting of probiotic supplementation with three strains of 10(9) colony-forming units of Lactobacillus (Lactobacillus reuteri, Lactobacillus casei subsp. rhamnosus, Lactobacillus plantarum) and a combined topical product of azelaic and Hydroxypinacolone Retinoate (HPR). Clinical evaluations and non-invasive imaging acquisitions using VISIA((R)) System, RCM, and D-OCT were performed at baseline, and after 4 and 12 weeks. The total number of clinically evident non-inflammatory lesions decreased during treatment from 11.5 to 7.3 (p < 0.05). There was also an evident reduction in microscopic acne features at RCM and D-OCT, such as the number of small bright follicles, large bright follicles and vascular threshold density at 300 mum and 500 mum depths. The types and extent of microscopic alterations in acne-prone skin patients may not be evident by clinical scores. Patients with low investigator global assessment (IGA) grades are a heterogeneous population, characterized by different microscopic skin features. Acne-prone skin is susceptible to treatment, and RCM and D-OCT imaging are sensitive tools to objectively monitor subclinical skin changes.
High Stability and Low Irritation of Retinol Propionate and Hydroxypinacolone Retinoate Supramolecular Nanoparticles with Effective Anti-Wrinkle Efficacy.[Pubmed:36986592]
Pharmaceutics. 2023 Feb 22;15(3):731.
Gravi-A nanoparticles, composed of retinyl propionate (RP) and Hydroxypinacolone Retinoate (HPR), were prepared by encapsulating the two using the high-pressure homogenization technique. The nanoparticles are effective in anti-wrinkle treatment with high stability and low irritation. We evaluated the effect of different process parameters on nanoparticle preparation. Supramolecular technology effectively produced nanoparticles with spherical shapes with an average size of 101.1 nm. The encapsulation efficiency was in the 97.98-98.35% range. The system showed a sustained release profile for reducing the irritation caused by Gravi-A nanoparticles. Furthermore, applying lipid nanoparticle encapsulation technology improved the transdermal efficiency of the nanoparticles, thereby allowing these to penetrate deep into the dermis layer to achieve precise and sustained release of active ingredients. Gravi-A nanoparticles can be extensively and conveniently used in cosmetics and other related formulations by direct application.
The synergistic effect of retinyl propionate and hydroxypinacolone retinoate on skin aging.[Pubmed:36762391]
J Cosmet Dermatol. 2023 Jul;22(7):2040-2049.
BACKGROUND: Aging is responsible for the majority of skin and soft tissue remolding in humans. Retinol and its derivatives or retinoids effectively intervene skin aging process. Nevertheless, retinoids usually induce skin intolerance, especially among the Chinese, and thus, their application to prevent skin aging is yet to be well accepted. The study of optimal composition and concentration of retinoids is necessary to offer strong antiaging efficacies with minimum irritations. Therefore, a better understanding of retinol and its derivatives is acutely needed to develop strategies to combat skin aging. OBJECTIVE: In this study, we aimed to determine the optimal ratio of two retinol derivatives-Hydroxypinacolone Retinoate (HPR) and retinyl propionate (RP) in terms of dermal remodeling and skin aging prevention-and to investigate their synergistic antiaging effects both in vitro and in vivo. METHODS: An in vitro human foreskin fibroblast (HFF-1) cell model was established to evaluate the cell viability of HPR and/or RP treatment. In addition, the antiaging and retinol receptor genes expressions in HFF-1 cells cotreated with HPR and RP were quantified. The in vivo adverse reaction evaluation of skincare serums containing various levels of retinol or the optimal HPR and RP combination termed Gravi-A was performed by 24 h patch tests in 33 subjects prior to the clinical research. Last but not the least, clinical research with 42 Chinese urban women was conducted to assess the in vivo antiaging efficacy of the skincare serum containing this optimal retinoid combination. RESULTS: The combination of HPR and RP at the weight ratio of 5:9 was shown to achieve the optimal in vitro antiaging performance. Coadministration of 5 mug/mL HPR and 9 mug/mL RP to HFF-1 cells promoted their proliferation at 24 h and synergistically enhanced the expressions of type IV collagen, CRBP-I, and RARB genes. In addition, the skincare serum containing HPR and RP combination at 5:9 weight ratio demonstrated superior in vivo anti-wrinkle and skin elasticity improvement benefits without any adverse reactions, while retinol in the same concentration exerted much higher adverse effect. Skin wrinkles, skin smoothness, TEWL, skin elasticity R2 and R5 were improved by 8.3%, 11.9%, 25.7%, 14.5%, and 22.6%, respectively, after 8-week use. CONCLUSION: Our results indicated the advanced antiaging effect of HPR and RP combination both in vitro and in vivo. In addition, little adverse effect was observed in this study, in comparison with retinol. This combination named as Gravi-A is a potential therapeutic strategy to prevent skin aging, especially for Chinese women.
Probiotic supplement combined with topical therapy in the treatment of mild to moderate acne: results from an Italian single centre interventional study.[Pubmed:36177779]
Ital J Dermatol Venerol. 2022 Dec;157(6):510-514.
BACKGROUND: Acne is a chronic inflammatory disease of the pilosebaceous unit resulting from different cofactors. The alteration of the skin microbiome has recently been revealed to play a role in acne pathogenesis. Concerns with side effects of available systemic treatment for acne resulted in a greater focus on topical therapies, such as topical azelaic acid which showed to be an effective and safe treatment option for acne. The aim of our study was to evaluate the efficacy of a new treatment protocol for acne based on an oral supplement composed of biotin and 3 strains of lactic ferments combined with a topical gel composed of azelaic acid, Hydroxypinacolone Retinoate, and alpha-hydroxy acids. METHODS: An Italian single-center interventional study was performed enrolling patients suffering from mild-to-moderate-acne. Patients were treated with a supplement based on biotin and 3 strains of lactic ferments, combined with a topical gel product (azelaic-acid, Hydroxypinacolone Retinoate, and alpha-hydroxy acids). All enrolled patients were scheduled for a total of 2 visits, a baseline visit (V0) and a follow-up visit after 60 days of treatment (V1). RESULTS: A total of 30 patients were enrolled in the study. Between V0 (baseline) and V1 (60 days), there was a reduction of 37.4% in the GAGS Score, 40.7% in the SEBUTAPEtm Score, and 18% in the TEWL Score, and an increment of 44% in the T-Blue Test Score. No cases of serious AEs were reported in our experience. CONCLUSIONS: Our results confirmed the promising therapeutic role of a probiotic supplement associated with topical therapy in the treatment of mild to moderate acne.
Retinoid stability and degradation kinetics in commercial cosmetic products.[Pubmed:33206444]
J Cosmet Dermatol. 2021 Jul;20(7):2350-2358.
BACKGROUND: Retinoids as dermatological agents are effective against acne, psoriasis, skin aging, and other skin conditions. However, their susceptibility to degradation is a limiting factor for their widespread use. OBJECTIVES: Within this study, we aimed to provide comprehensive and evidence-based information on retinoid stability and degradation kinetics in commercial cosmetics, focusing on different factors affecting their stability. METHODS: A validated HPLC-UV methodology was utilized for determination of the most common retinoids in cosmetics (retinol, retinyl palmitate, beta-carotene) and a newer promising retinoid (Hydroxypinacolone Retinoate). The stability of 16 retinoid derivatives in 12 commercial cosmetics was evaluated within 6 months of long-term and accelerated stability testing in addition to a one-week photostability study. Retinoid degradation in the tested formulations followed first-order kinetics, which was further applied to shelf-life prediction. RESULTS: Long-term and accelerated stability testing revealed retinoid instabilities in almost all products, resulting in a 0%-80% decline after 6 months at 25 degrees C and a 40%-100% decline at 40 degrees C, which were kinetically evaluated. Light degradation was more pronounced than temperature-induced degradation. Among the studied retinoids, the stability of the newer Hydroxypinacolone Retinoate was the most prominent. This study also identifies correlations between retinoid concentrations, price, formulation, and their stability in cosmetics. CONCLUSIONS: Retinoid instabilities were formulation-dependent and associated with lower contents than declared in some cosmetics. Retinoid chemical stability and physical stability in topical formulations need to be evaluated by real-time stability studies, instead of the more frequently used accelerated stability studies.
Efficacy and safety of a new topical gel formulation containing retinol encapsulated in glycospheres and hydroxypinacolone retinoate, an antimicrobial peptide, salicylic acid, glycolic acid and niacinamide for the treatment of mild acne: preliminary results of a 2-month prospective study.[Pubmed:32869963]
G Ital Dermatol Venereol. 2020 Oct;155(5):676-679.
BACKGROUND: Acne vulgaris is a common and chronic skin disease that impacts on physical and psychological perceptions. Combination therapy with topical retinoids and antimicrobial agent is considered the preferred approach for most of the subjects affected by mild-to-moderate acne. A correct therapeutic management should include a prolonged treatment to ensure therapeutic success and to prevent recurrences. The aim of this study was to evaluate the efficacy and tolerability of a new topical gel formulation that combines retinol encapsulated in glycospheres and Hydroxypinacolone Retinoate, associated with an anti-microbial peptide (BIOPEP-15) salicylic acid, glycolic acid, and niacinamide as monotherapy in mild acne vulgaris. METHODS: A 2-month prospective study was conducted at the Unit of Dermatology of the Federico II University. Twenty-five patients aged from 14 to 30 years with mild acne of the face (GAGS score
Quality control of retinoids in commercial cosmetic products.[Pubmed:32813932]
J Cosmet Dermatol. 2021 Apr;20(4):1166-1175.
BACKGROUND: Retinoids are widely used in different cosmetic products because of general improvement of skin appearance. However, retinoid concentration in cosmetics is restricted, and one particular form-retinoic acid, is banned in cosmetics due to safety reasons. AIMS: Within this study, we aimed to examine the quality of a considerable number of commercial retinoid cosmetic products in terms of their content and labeling, including also screening for the presence of retinoic acid. METHODS: An appropriate analytical methodology, based on HPLC-UV for the simultaneous determination of common retinoids, along with a screening method for retinoic acid, was developed and validated. Structural identity confirmation of the newer retinoid-Hydroxypinacolone Retinoate, was performed by LC-MS. RESULTS: Retinol and retinyl palmitate were most often found, in concentrations mostly below 0.3%, and up to 1.3% retinol equivalents. Determined contents deviated significantly from the quantitatively declared ones in seven products (0%-130%). In more than half of the tested products, inconsistencies between the contained and labeled retinoid were noticed. These products, as well as 14 additional anti-age cosmetics, were screened for retinoic acid, which was detected in two products. CONCLUSIONS: The obtained results from retinoids assay in commercial cosmetic products confirmed that the proposed method is appropriate for their routine analysis. The presence of retinoic acid in two products and determined retinoid contents above the Scientific Committee on Consumer Safety recommendations in 20% of the tested cosmetics reveal the need for their more strict regulation and quality control to ensure their efficacy and safety.
Efficacy and safety of a 12-month treatment with a combination of hydroxypinacolone retinoate and retinol glycospheres as maintenance therapy in acne patients after oral isotretinoin.[Pubmed:26889724]
G Ital Dermatol Venereol. 2017 Feb;152(1):13-17.
BACKGROUND: A correct therapeutic management of acne should include a maintenance therapy to prevent recurrences after discontinuing a successful treatment. The aim of this study is to investigate efficacy and safety of a 12-month maintenance treatment with a product, based on Retinsphere technology that combines retinol encapsulated in glycospheres and Hydroxypinacolone Retinoate (Biretix gel(R)), to control acne relapse after a treatment with oral isotretinoin (O.I.). METHODS: The study consisted of 2 phases: active treatment phase (AP) and maintenance phase (MP). In the AP, 40 consecutive patients with moderate facial acne were treated with O.I. until acne remission. Then, the patients entered in the MP and were treated with Biretix gel(R) once-daily for 12 months. The efficacy parameter was the relapse rate during MP. RESULTS: Thirty-nine patients completed the study. Relapse appeared in 6 patients (15.38%). The new product with Retinsphere technology was well tolerated and none of the subjects complained of adverse events. CONCLUSIONS: Our findings seems to provide favorable evidence of the efficacy and the safety of this new product in the maintenance treatment after O.I. in patient with moderate acne. The efficacy is maintain for a period as long as a year after O.I. suspension.
Treatment of mild to moderate acne with a fixed combination of hydroxypinacolone retinoate, retinol glycospheres and papain glycospheres.[Pubmed:25876142]
G Ital Dermatol Venereol. 2015 Apr;150(2):143-7.
AIM: A fixed combination of 0.1% Hydroxypinacolone Retinoate (synthetic esther of 9-cis-retinoic acid), 1% retinol in glycospheres and 2% papain in glycospheres in aqueous gel has been recently introduced into the Italian market in order to reduce the incidence and severity of irritant contact dermatitis caused by topical retinoids, without compromising their efficacy. Primary objectives of this sponsor-free, pilot, open, multicenter study were to evaluate the efficacy and tolerability of this gel in patients with comedonal-papular, mild to moderate acne of the face. METHODS: Ninety-eight Caucasian patients (28 males and 70 females), with an age ranging from 15 to 40 years, were treated with the gel once daily for 12 weeks. Acne severity and treatment efficacy were evaluated by means of the Global Acne Grading System (GAGS) and lesions count. RESULTS: Ninety-four patients were considered evaluable. A 41% mean reduction in the GAGS score was observed; a 40.8% mean reduction of total lesions was recorded; 15.3% of patients experienced mild to moderate local side effects (dryness, peeling, erythema, burning). No patients stopped the treatment because of these side effects. CONCLUSION: This study, based on a high number of evaluable patients, demonstrates that this fixed combination is an effective and safe option for the treatment of comedonal-papular, mild to moderate acne of the face. A controlled clinical study is necessary to confirm these data.


