HydroxyisogermafurenolideCAS# 20267-91-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 20267-91-8 | SDF | Download SDF |
| PubChem ID | 14038425.0 | Appearance | Powder |
| Formula | C15H20O3 | M.Wt | 248.32 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
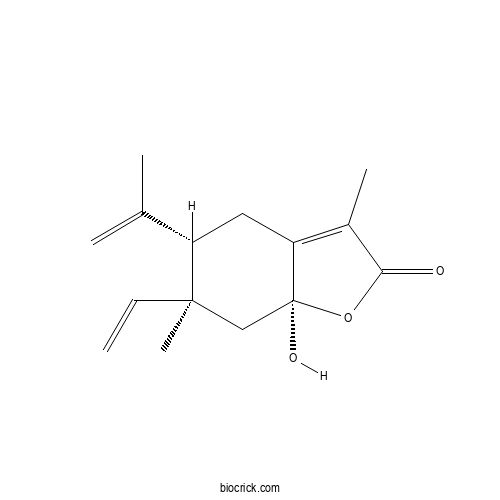
| Chemical Name | (5S,6S,7aS)-6-ethenyl-7a-hydroxy-3,6-dimethyl-5-prop-1-en-2-yl-5,7-dihydro-4H-1-benzofuran-2-one | ||
| SMILES | CC1=C2CC(C(CC2(OC1=O)O)(C)C=C)C(=C)C | ||
| Standard InChIKey | SZSKOUUYIBMAJD-GLQYFDAESA-N | ||
| Standard InChI | InChI=1S/C15H20O3/c1-6-14(5)8-15(17)12(7-11(14)9(2)3)10(4)13(16)18-15/h6,11,17H,1-2,7-8H2,3-5H3/t11-,14+,15-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Hydroxyisogermafurenolide Dilution Calculator

Hydroxyisogermafurenolide Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.0271 mL | 20.1353 mL | 40.2706 mL | 80.5412 mL | 100.6765 mL |
| 5 mM | 0.8054 mL | 4.0271 mL | 8.0541 mL | 16.1082 mL | 20.1353 mL |
| 10 mM | 0.4027 mL | 2.0135 mL | 4.0271 mL | 8.0541 mL | 10.0677 mL |
| 50 mM | 0.0805 mL | 0.4027 mL | 0.8054 mL | 1.6108 mL | 2.0135 mL |
| 100 mM | 0.0403 mL | 0.2014 mL | 0.4027 mL | 0.8054 mL | 1.0068 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 14-Deoxy-11-oxoandrographolide
Catalog No.:BCX0926
CAS No.:42895-57-8
- MogrosideIIA
Catalog No.:BCX0925
CAS No.:1613527-65-3
- Pseudojervine
Catalog No.:BCX0924
CAS No.:36069-05-3
- BoeravinoneA
Catalog No.:BCX0923
CAS No.:114567-33-8
- D-Pyroglutamicacid
Catalog No.:BCX0922
CAS No.:4042-36-8
- VanillylButylEther
Catalog No.:BCX0921
CAS No.:82654-98-6
- SalicylaldehydeAzine
Catalog No.:BCX0920
CAS No.:959-36-4
- 4-Dimethylaminobenzaldehyde
Catalog No.:BCX0919
CAS No.:100-10-7
- Cinnamylacetate
Catalog No.:BCX0918
CAS No.:103-54-8
- 8-Methoxyquinoline-2-carbaldehyde
Catalog No.:BCX0917
CAS No.:103854-64-4
- PALATINOSE
Catalog No.:BCX0916
CAS No.:13718-94-0
- Chlorine6
Catalog No.:BCX0915
CAS No.:19660-77-6
- (+)-Pinoresinolmonomethylether4-O-β-D-glucoside
Catalog No.:BCX0928
CAS No.:74957-57-6
- 3-(3-hydroxylphenyl)propanol
Catalog No.:BCX0929
CAS No.:621-54-5
- PhysalinF
Catalog No.:BCX0930
CAS No.:57517-46-1
- Cepharanoline
Catalog No.:BCX0931
CAS No.:27686-34-6
- Nicotinamidemononucleotide
Catalog No.:BCX0932
CAS No.:1094-61-7
- 2-Methoxybenzoicacid
Catalog No.:BCX0933
CAS No.:529-75-9
- Hydroxypinacolone Retinoate
Catalog No.:BCX0934
CAS No.:893412-73-2
- Coumarin 343
Catalog No.:BCX0935
CAS No.:55804-65-4
- LINOLELAIDICACIDMETHYLESTER
Catalog No.:BCX0936
CAS No.:2566-97-4
- Ginno
Catalog No.:BCX0937
CAS No.:2606-50-0
- β-Elemene
Catalog No.:BCX0938
CAS No.:515-13-9
- PhlorigidosideC
Catalog No.:BCX0939
CAS No.:276691-32-8
[Quality evaluation of Curcumae Radix from different origins based on UPLC characteristic chromatogram, multicomponent content, and chemometrics].[Pubmed:35718518]
Zhongguo Zhong Yao Za Zhi. 2022 Jun;47(11):2964-2974.
In this study, UPLC was used to establish the characteristic chromatograms of Curcumae Radix from different origins(LSYJ, WYJ, HSYJ, and GYJ) and the content determination method of 11 chemical components. The evaluation of characteristic chromatogram similarity, cluster analysis(CA), principal component analysis(PCA), and orthogonal partial least square discriminant analysis(OPLS-DA) were combined to evaluate the quality of Curcumae Radix from four origins. LSYJ, WYJ, HSYJ, and GYJ showed 15, 17, 15, and 10 characteristic peaks, respectively, and 8 of the peaks were identified. The characteristic chromatograms of Curcumae Radix samples(except for GYJ07) from the same origin showed the similarity greater than 0.854. The 11 chemical components had different content among the samples from four origins. Curcumenol, furanodienone, and isocurcumenol were rich in LSYJ; Hydroxyisogermafurenolide, curdione, and furanodiene had high content in WYJ; gemacrone, beta-elemene, bisdemethoxycurcumin, demethoxycurcumin, and curcumin were rich in HSYJ; all the components had low content in GYJ. The chemometric analysis showed that CA, PCA, and OPLS-DA could accurately classify the four origins of Curcumae Radix into four categories, and five different quality markers, namely furanodienone, curcumenol, curdione, Hydroxyisogermafurenolide, and furanodiene, were screened out by OPLS-DA. UPLC in combination with multicomponent content determination is simple, rapid, reproducible, and specific, which can provide reference for the quality control and identification of Curcumae Radix from four origins.
[Chemical constituents of sesquiterpenes from Chloranthus multistachys].[Pubmed:34467726]
Zhongguo Zhong Yao Za Zhi. 2021 Aug;46(16):4145-4149.
With repeated silica gel, octadecyl silica(ODS), and Sephadex LH-20 column chromatography, normal-phase and reverse-phase high performance liquid chromatography(HPLC), etc., a pair of new enantiomers and 5 known compounds were separated from the 95% ethanol extract of Chloranthus multistachys. These compounds were identified by the nuclear magnetic resonance spectroscopy(including 1 D-NMR and 2 D-NMR), single-crystal X-ray diffraction, circular dichroism(CD) spectroscopy, mass spectrometry(MS), and some other methods as(1R,4R,5R,8S,10R)-chloraeudolide H(1 a),(1S,4S,5S,8R,10S)-chloraeudolide H(1 b), Hydroxyisogermafurenolide(2), 4alpha-hydroxy-5alpha,8beta(H)-eudesm-7(11)-en-8,12-olide(3), chloraniolide A(4), chlorantene D(5), 4alpha,8beta-dihydroxy-5alpha(H)-eudesm-7(11)-en-8,12-olide(6). Compounds 1 a and 1 b are a pair of new eudesmane-type sesquiterpene enantiomers, and compounds 2-4 were isolated from C. multistachys for the first time.
Investigation of Antiplasmodial Effects of Terpenoid Compounds Isolated from Myrrh.[Pubmed:32365391]
Planta Med. 2020 Jun;86(9):643-654.
As part of our ongoing search for antiprotozoal natural products from plants, we examined different resins from the Burseraceae family. The dichloromethane extract obtained from myrrh, the oleo-gum-resin of Commiphora species, showed promising in vitro activity against Plasmodium falciparum with an IC(50) value of 1 microg/mL. Bioactivity-guided fractionation led to the isolation and characterization of 18 sesquiterpenoids, namely, beta-elemene (1: ), elemyl acetate (2: ), curzerenone (3: ), 8-Hydroxyisogermafurenolide (4: ), 2-methoxyisogermafurenolide (5: ), 8-epi-2-methoxyisogermafurenolide (6: ), furanodienone (7: ), 1(10)Z,4Z-furanodien-6-one (8: ), rel-2R-methyl-5S-acetoxy-4R-furanogermacr-1(10)Z-en-6-one (9: ), (1(10)E)-2-methoxy-8,12-epoxygermacra-1(10),7,11-trien-6-one (10: ), 2R-methoxyfuranodiene (11: ), 2-acetyloxyglechomanolide (12: ), 8-epi-2-acetyloxyglechomanolide (13: ), (1R,2R,4S)-1,2-epoxyfuranogermacr-10(15)-en-6-one (14: ), hydroxylindestrenolide (15: ), isohydroxylindestrenolide (16: ), myrrhone (17: ), and myrrhterpenoid O (18: ). Moreover, nine (nor-)triterpenoids were isolated: mansumbinol (19: ), mansumbinol epoxide (20: ), mansumbinone (21: ), mansumbin-13(17)-en-3,16-dione (22: ), 3,4-seco-mansumbinoic acid (23: ), rel-20S-hydroxy-dammar-24-en-3,16-dione (24: ), rel-(16S,20S)-dihydroxydammar-24-en-3-one (25: ), cycloart-24-en-1alpha,2alpha,3beta-triol (26: ), and 3beta-isovaleroyloxycycloart-24-en-1alpha,2alpha-diol (27: ). All compounds were identified by MS and NMR spectroscopic analyses. To the best of our knowledge, compounds 5, 6, 12, 13, 16, 18: , and 20: are reported for the first time. All isolated compounds were tested in vitro for activity against P. falciparum and cytotoxicity. The sesquiterpene 7: and the triterpene 25: were the most active compounds found in this study with IC(50) values of 7.4 and 2.8 microM, respectively.
Total synthesis, structural revision and biological evaluation of gamma-elemene-type sesquiterpenes.[Pubmed:30303229]
Org Biomol Chem. 2018 Oct 31;16(42):7843-7850.
Total synthesis and absolute configuration confirmation of gamma-elemene-type sesquiterpenes, which possess vast potential for biological activities, was investigated based on a convergent synthetic strategy. A key intermediate with all functional groups of this family of natural products was accessed by an intermolecular aldol reaction and then an acetylation of a known ketone (12) derived from commercially available verbenone. The versatile intermediate can be easily transformed into structurally different gamma-elemene-type sesquiterpenes based on control of base-promoted cyclization manipulation in different solvents. The utility of this robust approach is illustrated by the first syntheses of elema-1,3,7(11),8-tetraen-8,12-lactam (4') and 8beta-methoxy-isogermafurenolide (6a), as well as the syntheses of elem-1,3,7,8-tetraen-8,12-olide (3) and Hydroxyisogermafurenolide (5) in only 6 or 7 steps. In addition, the structure of the reported 5betaH-elem-1,3,7,8-tetraen-8,12-olide (1) was revised as elem-1,3,7,8-tetraen-8,12-olide (3) by comparison of their identified datum, and the absolute configuration of elema-1,3,7(11),8-tetraen-8,12-lactam was confirmed as 4'. Furthermore, the inhibitory effect of all synthesized natural compounds and their natural analogues on cancer cell proliferation was evaluated. Among them compounds 3, 4 and 4' were found to possess potent inhibitory activity against Kasumi-1 and Pfeiffer. Meanwhile, preliminary structure-activity relationships for these compounds are discussed.
Sesquiterpene lactones from the root tubers of Lindera aggregata.[Pubmed:19639966]
J Nat Prod. 2009 Aug;72(8):1497-501.
Phytochemical investigation of the root tubers of Lindera aggregata resulted in the isolation of five new sesquiterpene lactones, linderagalactones A-E (1-5), along with eight known sesquiterpenoids, 3-eudesmene-1beta,11-diol, hydroxylindestenolide, strychnistenolide, Hydroxyisogermafurenolide, atractylenolide III, linderane, neolinderalactone, and linderalactone. The structures and relative configurations of 1-5 were determined by spectroscopic methods, especially HRESIMS and 2D NMR techniques. The absolute configurations of 1-4 were defined by comparison of quantum chemical TDDFT calculated and experimental ECD spectra. Linderagalactone A (1) is a halogenated sesquiterpene lactone possessing a unique rearranged carbon skeleton. Linderagalactone E (5), linderane, hydroxylindestenolide, and linderalactone showed hepatoprotective activity against H2O2-induced oxidative damages on HepG2 cells with EC(50) values of 67.5, 167.0, 42.4, and 98.0 microM, respectively.


