Chlorine6CAS# 19660-77-6 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 19660-77-6 | SDF | Download SDF |
| PubChem ID | 5479494.0 | Appearance | Powder |
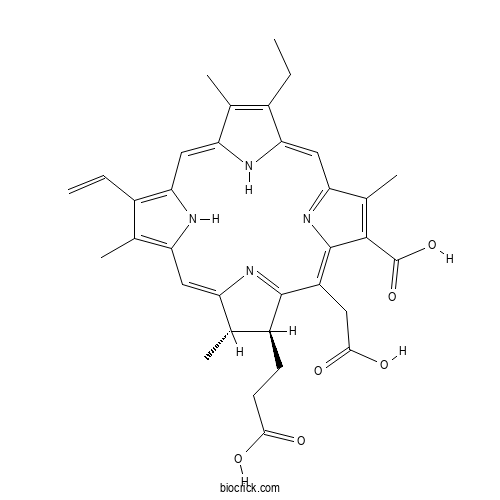
| Formula | C34H36N4O6 | M.Wt | 596.67 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (17S,18S)-18-(2-carboxyethyl)-20-(carboxymethyl)-12-ethenyl-7-ethyl-3,8,13,17-tetramethyl-17,18,22,23-tetrahydroporphyrin-2-carboxylic acid | ||
| SMILES | CCC1=C(C2=CC3=C(C(=C(N3)C=C4C(C(C(=N4)C(=C5C(=C(C(=N5)C=C1N2)C)C(=O)O)CC(=O)O)CCC(=O)O)C)C)C=C)C | ||
| Standard InChIKey | OYINILBBZAQBEV-UWJYYQICSA-N | ||
| Standard InChI | InChI=1S/C34H36N4O6/c1-7-19-15(3)23-12-25-17(5)21(9-10-29(39)40)32(37-25)22(11-30(41)42)33-31(34(43)44)18(6)26(38-33)14-28-20(8-2)16(4)24(36-28)13-27(19)35-23/h7,12-14,17,21,35-36H,1,8-11H2,2-6H3,(H,39,40)(H,41,42)(H,43,44)/t17-,21-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Chlorine6 Dilution Calculator

Chlorine6 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.676 mL | 8.3798 mL | 16.7597 mL | 33.5194 mL | 41.8992 mL |
| 5 mM | 0.3352 mL | 1.676 mL | 3.3519 mL | 6.7039 mL | 8.3798 mL |
| 10 mM | 0.1676 mL | 0.838 mL | 1.676 mL | 3.3519 mL | 4.1899 mL |
| 50 mM | 0.0335 mL | 0.1676 mL | 0.3352 mL | 0.6704 mL | 0.838 mL |
| 100 mM | 0.0168 mL | 0.0838 mL | 0.1676 mL | 0.3352 mL | 0.419 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Climbazole
Catalog No.:BCX0914
CAS No.:38083-17-9
- Oxysophoridine
Catalog No.:BCX0913
CAS No.:54809-74-4
- Graniline
Catalog No.:BCX0912
CAS No.:40737-97-1
- Dehydrocostuslactone
Catalog No.:BCX0911
CAS No.:74299-48-2
- Sodiumnewhouttuyfonate
Catalog No.:BCX0910
CAS No.:83766-73-8
- Isomaltotriose
Catalog No.:BCX0909
CAS No.:3371-50-4
- Fructo-oligosaccharideDP8/GF7
Catalog No.:BCX0908
CAS No.:62512-21-4
- 1-Kestoheptaose
Catalog No.:BCX0907
CAS No.:62512-20-3
- Maltooctaose
Catalog No.:BCX0906
CAS No.:6156-84-9
- 5-Hydroxytryptophan
Catalog No.:BCX0905
CAS No.:114-03-4
- 8-Methoxyquinoline-2-carboxylicacid
Catalog No.:BCX0904
CAS No.:21141-35-5
- Sodium taurolithocholate
Catalog No.:BCX0903
CAS No.:6042-32-6
- PALATINOSE
Catalog No.:BCX0916
CAS No.:13718-94-0
- 8-Methoxyquinoline-2-carbaldehyde
Catalog No.:BCX0917
CAS No.:103854-64-4
- Cinnamylacetate
Catalog No.:BCX0918
CAS No.:103-54-8
- 4-Dimethylaminobenzaldehyde
Catalog No.:BCX0919
CAS No.:100-10-7
- SalicylaldehydeAzine
Catalog No.:BCX0920
CAS No.:959-36-4
- VanillylButylEther
Catalog No.:BCX0921
CAS No.:82654-98-6
- D-Pyroglutamicacid
Catalog No.:BCX0922
CAS No.:4042-36-8
- BoeravinoneA
Catalog No.:BCX0923
CAS No.:114567-33-8
- Pseudojervine
Catalog No.:BCX0924
CAS No.:36069-05-3
- MogrosideIIA
Catalog No.:BCX0925
CAS No.:1613527-65-3
- 14-Deoxy-11-oxoandrographolide
Catalog No.:BCX0926
CAS No.:42895-57-8
- Hydroxyisogermafurenolide
Catalog No.:BCX0927
CAS No.:20267-91-8
Bionic natural small molecule co-assemblies towards targeted and synergistic Chemo/PDT/CDT.[Pubmed:37161611]
Biomater Res. 2023 May 9;27(1):43.
BACKGROUND: Multi-component nano-delivery systems based on chemotherapy (chemo)- photodynamic therapy (PDT)- chemodynamic therapy (CDT) have gained increased attention as a promising strategy to improve clinical outcomes in cancer treatment. However, there remains a challenge in developing biodegradable, biocompatible, less toxic, yet highly efficient multicomponent nanobased drug delivery systems (DDS). Here, our study presents the screening and development of a novel DDS based on co-assemblies natural small molecule (NSMs). These molecules (oleanolic acid, and betulinic acid) are combined with photosensitizers Chlorine6 (Ce6) and Cu(2+) that are encapsulated by tumor cell membranes. This nanocarrier encapsulated in tumor cell membranes achieved good tumor targeting and a significant improvement in tumor accumulation. METHODS: A reprecipitation method was used to prepare the co-assembled nanocarrier, followed by the introduction of Cu(2 +) into the DDS (OABACe6 NPs). Then, by wrapping the surface of NPs with the cell membranes of 4T1 which is a kind of mouse breast cancer cells (CM@OABACe6/Cu NPs). and analysis of its structure and size distribution with UV-Vis, XPS, FT-IR, SEM, TEM, and DLS. The synergistic effects of in vitro chemotherapy, CDT and PDT and targeting were also validated by cellular and animal studies. RESULTS: It was shown that CM@OABACe6/Cu NPs achieved good tumor targeting and a significant improvement in tumor accumulation. In the composite nano-assembly, the NSMs work together with the Ce6 to provide effective and safe chemo and PDT. Moreover, the effect of reduced PDT due to the depletion of reactive oxygen species (ROS) by excess glutathione (GSH) in the tumor can be counteracted when Cu(2 +) is introduced. More importantly, it also confers CDT through a Fenton-like catalytic reaction with H(2)O overexpressed at the tumor site. CONCLUSIONS: By constructing CM@OABACe6/Cu NPs with homologous targeting, we create a triple synergistic platform for cancer therapy using PDT, chemo, and CDT. We propose here a novel combinatorial strategy for designing more naturally co-assembled small molecules, especially for the development of multifunctional synergistic therapies that utilize NSMs.
DNAzyme-Assisted Nano-Herb Delivery System for Multiple Tumor Immune Activation.[Pubmed:36156383]
Small. 2022 Nov;18(45):e2203942.
As a promising therapeutic strategy against cancer, immunotherapy faces critical challenges, especially in solid tumors. Immune checkpoint blockade therapy, particularly blocking the interaction of the programmed cell death 1 (PD1)-PD1 ligand 1 (PD-L1) axis, can reverse the suppression of T cells so as to destroy tumor cells and exert antitumor effects. Here, a strategy of multiple activation of immune pathways is developed, to provide supporting evidence for potential antitumor therapies. Briefly, a pH/glutathione responsive drug-loading hollow-manganese dioxide (H-MnO(2) )-based Chlorine6 (Ce6)-modified DNAzyme therapeutic nanosystem for the combination of gene therapy and immunotherapy is established. The H-MnO(2) nanoparticles could efficiently deliver the DNAzyme and glycyrrhizic acid (GA) to enhance the tumor target effects. In the tumor microenvironments, the biodegradation of H-MnO(2) via pH-induced hydrolyzation allows the release of guest DNAzyme payloads and host Mn(2+) ions, which serve as PD-L1 mRNA-targeting reagent and require DNAzyme cofactors for activating gene therapy. In addition, Mn(2+) is also associated with the immune activation of thcGAS-STING pathway. Auxiliary photosensitizers Ce6 and GA could produce reactive oxygen species, resulting in immunogenic cell death. Overall, this study provides a general strategy for targeted gene inhibition and GA release, which is valuable for the development of potential tumor immunotherapies.
NIR-Triggered and ROS-Boosted Nanoplatform for Enhanced Chemo/PDT/PTT Synergistic Therapy of Sorafenib in Hepatocellular Carcinoma.[Pubmed:36125619]
Nanoscale Res Lett. 2022 Sep 20;17(1):92.
Although being the first-line treatment of advanced hepatocellular carcinoma (HCC), sorafenib (SOR) outcome is limited due to drug resistance and low tumor accumulation. Herein, with MnO(2) as photothermal agent and Chlorine6 (Ce6) as photosensitizer, a tumor-targeting and NIR-triggered multifunctional nanoplatform loading sorafenib (MnO(2)-SOR-Ce6@PDA-PEG-FA, MSCPF) was constructed. Owing to oxygen generator MnO(2), MSCPF could generate excessive ROS, thus can alleviate tumor hypoxia and improve sorafenib accumulation in cancer cells. Besides, ROS production further strengthens Ce6-mediated PDT and PDA-mediated PTT. By exploiting these features, MSCPF exhibited excellent antitumor effects on HCC in the in vitro and in vivo studies, compared to solo sorafenib or PDT/PTT treatment. Further mechanism experiments suggested that MSCPF could inhibit P-gp expression and induce ferroptosis via deactivation of GPX4 and SLC7A11, which ultimately enhanced the antitumor efficacy of SOR. In summary, our work highlights a promising NIR-triggered and ROS-boosted nanoplatform for enhanced chemo/PDT/PTT synergistic therapy of SOR in HCC treatment.


