8-Methoxyquinoline-2-carbaldehydeCAS# 103854-64-4 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 103854-64-4 | SDF | Download SDF |
| PubChem ID | 1548870.0 | Appearance | Powder |
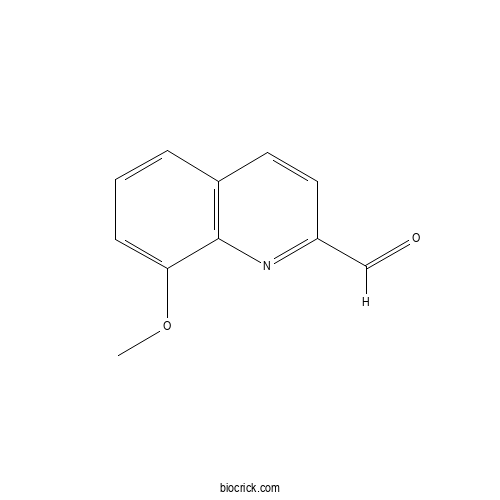
| Formula | C11H9NO2 | M.Wt | 187.19 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 8-methoxyquinoline-2-carbaldehyde | ||
| SMILES | COC1=CC=CC2=C1N=C(C=C2)C=O | ||
| Standard InChIKey | WXFAZCYUMXZXBA-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C11H9NO2/c1-14-10-4-2-3-8-5-6-9(7-13)12-11(8)10/h2-7H,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

8-Methoxyquinoline-2-carbaldehyde Dilution Calculator

8-Methoxyquinoline-2-carbaldehyde Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.3422 mL | 26.7108 mL | 53.4217 mL | 106.8433 mL | 133.5541 mL |
| 5 mM | 1.0684 mL | 5.3422 mL | 10.6843 mL | 21.3687 mL | 26.7108 mL |
| 10 mM | 0.5342 mL | 2.6711 mL | 5.3422 mL | 10.6843 mL | 13.3554 mL |
| 50 mM | 0.1068 mL | 0.5342 mL | 1.0684 mL | 2.1369 mL | 2.6711 mL |
| 100 mM | 0.0534 mL | 0.2671 mL | 0.5342 mL | 1.0684 mL | 1.3355 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- PALATINOSE
Catalog No.:BCX0916
CAS No.:13718-94-0
- Chlorine6
Catalog No.:BCX0915
CAS No.:19660-77-6
- Climbazole
Catalog No.:BCX0914
CAS No.:38083-17-9
- Oxysophoridine
Catalog No.:BCX0913
CAS No.:54809-74-4
- Graniline
Catalog No.:BCX0912
CAS No.:40737-97-1
- Dehydrocostuslactone
Catalog No.:BCX0911
CAS No.:74299-48-2
- Sodiumnewhouttuyfonate
Catalog No.:BCX0910
CAS No.:83766-73-8
- Isomaltotriose
Catalog No.:BCX0909
CAS No.:3371-50-4
- Fructo-oligosaccharideDP8/GF7
Catalog No.:BCX0908
CAS No.:62512-21-4
- 1-Kestoheptaose
Catalog No.:BCX0907
CAS No.:62512-20-3
- Maltooctaose
Catalog No.:BCX0906
CAS No.:6156-84-9
- 5-Hydroxytryptophan
Catalog No.:BCX0905
CAS No.:114-03-4
- Cinnamylacetate
Catalog No.:BCX0918
CAS No.:103-54-8
- 4-Dimethylaminobenzaldehyde
Catalog No.:BCX0919
CAS No.:100-10-7
- SalicylaldehydeAzine
Catalog No.:BCX0920
CAS No.:959-36-4
- VanillylButylEther
Catalog No.:BCX0921
CAS No.:82654-98-6
- D-Pyroglutamicacid
Catalog No.:BCX0922
CAS No.:4042-36-8
- BoeravinoneA
Catalog No.:BCX0923
CAS No.:114567-33-8
- Pseudojervine
Catalog No.:BCX0924
CAS No.:36069-05-3
- MogrosideIIA
Catalog No.:BCX0925
CAS No.:1613527-65-3
- 14-Deoxy-11-oxoandrographolide
Catalog No.:BCX0926
CAS No.:42895-57-8
- Hydroxyisogermafurenolide
Catalog No.:BCX0927
CAS No.:20267-91-8
- (+)-Pinoresinolmonomethylether4-O-β-D-glucoside
Catalog No.:BCX0928
CAS No.:74957-57-6
- 3-(3-hydroxylphenyl)propanol
Catalog No.:BCX0929
CAS No.:621-54-5
A new ratiometric switch "two-way" detects hydrazine and hypochlorite via a "dye-release" mechanism with a PBMC bioimaging study.[Pubmed:36053209]
Phys Chem Chem Phys. 2022 Sep 14;24(35):20941-20952.
A new ratiometric fluorescent probe (E)-2-(benzo[d]thiazol-2-yl)-3-(8-methoxyquinolin-2-yl)acrylonitrile (HQCN) was synthesised by the perfect blending of quinoline and a 2-benzothiazoleacetonitrile unit. In a mixed aqueous solution, HQCN reacts with hydrazine (N(2)H(4)) to give a new product 2-(hydrazonomethyl)-8-methoxyquinoline along with the liberation of the 2-benzothiazoleacetonitrile moiety. In contrast, the reaction of hypochlorite ions (OCl(-)) with the probe gives 8-Methoxyquinoline-2-carbaldehyde. In both cases, the chemodosimetric approaches of hydrazine and hypochlorite selectively occur at the olefinic carbon but give two different products with two different outputs, as observed from the fluorescence study exhibiting signals at 455 nm and 500 nm for hydrazine and hypochlorite, respectively. A UV-vis spectroscopy study also depicts a distinct change in the spectrum of HQCN in the presence of hydrazine and hypochlorite. The hydrazinolysis of HQCN exhibits a prominent chromogenic as well as ratiometric fluorescence change with a 165 nm left-shift in the fluorescence spectrum. Similarly, the probe in hand (HQCN) can selectively detect hypochlorite in a ratiometric manner with a shift of 120 nm, as observed from the fluorescence emission spectra. HQCN can detect hydrazine and OCl(-) as low as 2.25 x 10(-8) M and 3.46 x 10(-8) M, respectively, as evaluated from the fluorescence experiments again. The excited state behaviour of the probe HQCN and the chemodosimetric products with hydrazine and hypochlorite are studied by the nanosecond time-resolved fluorescence technique. Computational studies (DFT and TDDFT) with the probe and the hydrazine and hypochlorite products were also performed. The observations made in the fluorescence imaging studies with human blood cells manifest that HQCN can be employed to monitor hydrazine and OCl(-) in human peripheral blood mononuclear cells (PBMCs). It is indeed a rare case that the single probe HQCN is found to be successfully able to detect hydrazine and hypochlorite in PBMCs, with two different outputs.


