14-Deoxy-11-oxoandrographolideCAS# 42895-57-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 42895-57-8 | SDF | Download SDF |
| PubChem ID | 9975052.0 | Appearance | Powder |
| Formula | C20H28O5 | M.Wt | 348.44 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
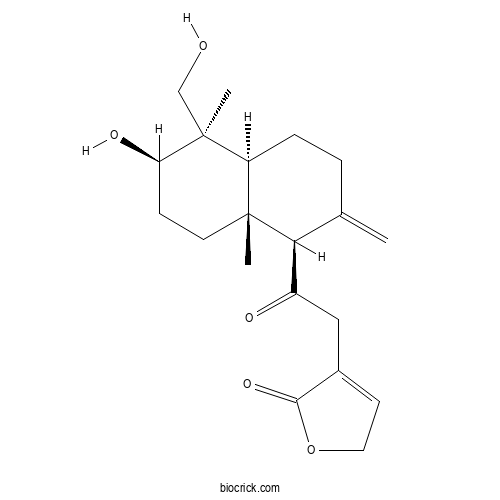
| Chemical Name | 4-[2-[(1R,4aS,5R,6R,8aR)-6-hydroxy-5-(hydroxymethyl)-5,8a-dimethyl-2-methylidene-3,4,4a,6,7,8-hexahydro-1H-naphthalen-1-yl]-2-oxoethyl]-2H-furan-5-one | ||
| SMILES | CC12CCC(C(C1CCC(=C)C2C(=O)CC3=CCOC3=O)(C)CO)O | ||
| Standard InChIKey | WZHWNAKOQGIEEB-NDLGOLERSA-N | ||
| Standard InChI | InChI=1S/C20H28O5/c1-12-4-5-15-19(2,8-6-16(23)20(15,3)11-21)17(12)14(22)10-13-7-9-25-18(13)24/h7,15-17,21,23H,1,4-6,8-11H2,2-3H3/t15-,16+,17-,19+,20-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

14-Deoxy-11-oxoandrographolide Dilution Calculator

14-Deoxy-11-oxoandrographolide Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.8699 mL | 14.3497 mL | 28.6993 mL | 57.3987 mL | 71.7484 mL |
| 5 mM | 0.574 mL | 2.8699 mL | 5.7399 mL | 11.4797 mL | 14.3497 mL |
| 10 mM | 0.287 mL | 1.435 mL | 2.8699 mL | 5.7399 mL | 7.1748 mL |
| 50 mM | 0.0574 mL | 0.287 mL | 0.574 mL | 1.148 mL | 1.435 mL |
| 100 mM | 0.0287 mL | 0.1435 mL | 0.287 mL | 0.574 mL | 0.7175 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- MogrosideIIA
Catalog No.:BCX0925
CAS No.:1613527-65-3
- Pseudojervine
Catalog No.:BCX0924
CAS No.:36069-05-3
- BoeravinoneA
Catalog No.:BCX0923
CAS No.:114567-33-8
- D-Pyroglutamicacid
Catalog No.:BCX0922
CAS No.:4042-36-8
- VanillylButylEther
Catalog No.:BCX0921
CAS No.:82654-98-6
- SalicylaldehydeAzine
Catalog No.:BCX0920
CAS No.:959-36-4
- 4-Dimethylaminobenzaldehyde
Catalog No.:BCX0919
CAS No.:100-10-7
- Cinnamylacetate
Catalog No.:BCX0918
CAS No.:103-54-8
- 8-Methoxyquinoline-2-carbaldehyde
Catalog No.:BCX0917
CAS No.:103854-64-4
- PALATINOSE
Catalog No.:BCX0916
CAS No.:13718-94-0
- Chlorine6
Catalog No.:BCX0915
CAS No.:19660-77-6
- Climbazole
Catalog No.:BCX0914
CAS No.:38083-17-9
- Hydroxyisogermafurenolide
Catalog No.:BCX0927
CAS No.:20267-91-8
- (+)-Pinoresinolmonomethylether4-O-β-D-glucoside
Catalog No.:BCX0928
CAS No.:74957-57-6
- 3-(3-hydroxylphenyl)propanol
Catalog No.:BCX0929
CAS No.:621-54-5
- PhysalinF
Catalog No.:BCX0930
CAS No.:57517-46-1
- Cepharanoline
Catalog No.:BCX0931
CAS No.:27686-34-6
- Nicotinamidemononucleotide
Catalog No.:BCX0932
CAS No.:1094-61-7
- 2-Methoxybenzoicacid
Catalog No.:BCX0933
CAS No.:529-75-9
- Hydroxypinacolone Retinoate
Catalog No.:BCX0934
CAS No.:893412-73-2
- Coumarin 343
Catalog No.:BCX0935
CAS No.:55804-65-4
- LINOLELAIDICACIDMETHYLESTER
Catalog No.:BCX0936
CAS No.:2566-97-4
- Ginno
Catalog No.:BCX0937
CAS No.:2606-50-0
- β-Elemene
Catalog No.:BCX0938
CAS No.:515-13-9
The Natural Ligand for Metalloproteinase-A Multifaceted Drug Target.[Pubmed:34997448]
Appl Biochem Biotechnol. 2022 Apr;194(4):1716-1739.
Metalloproteinase is one of the key components of Russell viper venom and it is the root cause of edema, blood coagulation, local tissue damage, hemorrhage, and inflammation during snakebite envenoming. Hence, finding a suitable metalloproteinase inhibitor from natural source will be of great biological importance in mitigating pathological effects. In this current study, we employed computational analysis to examine the inhibition of metalloproteinase by phytochemicals present in Andrographis paniculata. Molecular docking studies revealed interaction of A. paniculata phytochemicals with the catalytic M domain's active site amino acid residues, namely ASN203, ARG293, PHE203, LEU206, LYS199, and ALA122, similar to that of the reference compound Batimastat. 14-acetylandrographolide, 14-deoxy-11,12 didehydroandrographolide, Andrograpanin, Isoandrographolide, and 14-Deoxy-11-oxoandrographolide displayed high binding energy and inhibition against the metalloproteinase. Molecular dynamic simulation analysis revealed less root mean square fluctuation of amino acid residues of metalloproteinase-14-acetylandrographolide complex than metalloproteinase-Batimastat complex indicating the high stability for metalloproteinase with the phytochemical. In silico analysis of parameters like ADME properties and drug-likeness of the phytochemicals exhibited good pharmacokinetic properties. Ligand-based virtual screening of phytochemicals to identify similarity to FDA-approved drugs and identification of their possible targets were also performed. The outcome of the current study strengthens the significance of these phytochemicals as promising lead candidates for the treatment of snakebite envenomation. Moreover, the study also encourages the in vivo and in vitro evaluation of the phytochemicals to validate the computational findings.
Structural basis for complementary and alternative medicine: Phytochemical interaction with non-structural protein 2 protease-a reverse engineering strategy.[Pubmed:25491534]
Chin J Integr Med. 2015 Jun;21(6):445-52.
OBJECTIVE: To understand the druggability of the bioactive compounds from traditional herbal formulations "Nilavembu Kudineer" and "Swasthya Raksha Amruta Peya" to heal chikungunya virus (CHIKV) infection. METHODS: The efficiency of twenty novel chemical entities from "Nilavembu Kudineer" and "Swasthya Raksha Amruta Peya" to inhibit CHIKV infection in silico were evaluated. Ligands were prepared using Ligprep module of Schrodinger. Active site was identified using SiteMap program. Grid box was generated using receptor grid generation wizard. Molecular docking was carried out using Grid Based Ligand Docking with Energetics (GLIDE) program. RESULTS: Molecular docking studies showed that among twenty compounds, andrographoside, deoxyandrographoside, neoandrographolide, 14-Deoxy-11-oxoandrographolide, butoxone and oleanolic acid showed GLIDE extra precision (XP) score of -9.10, -8.72, -8.25, -7.38, -7.28 and -7.01, respectively which were greater than or comparable with chloroquine (reference compound) XP score (-7.08) and were found to interact with the key residues GLU 1043, LYS 1045, GLY 1176, LEU 1203, HIS 1222 and LYS 1239 which were characteristic functional unit crucial for replication of CHIKV. CONCLUSION: The binding affinity and the binding mode of chemical entities taken from herbal formulations with non-structural protein 2 protease were understood and our study provided a novel strategy in the development and design of drugs for CHIKV infection.
Delivery in vivo of 14-deoxy-11-oxoandrographolide, an antileishmanial agent, by different drug carriers.[Pubmed:22900306]
Indian J Biochem Biophys. 2003 Jun;40(3):169-74.
An antileishmanial compound, 14-deoxy-11-oxo-andrographolide, a derivative of andrographlide, isolated from the Indian medicinal plant Andrographis paniculata was evaluated for efficacy in free form and in different vesicular delivery modes on hamster model of Leishmaniasis. The subcutaneous injection of free drug reduced the spleen parasite load by 39%, whereas for drug incorporated in liposomes, niosomes and microspheres, reductions in the parasite load were 78%, 91% and 59%, respectively. Moreover, the drug in various delivery modes, particularly in liposomal and niosomal forms, showed no apparent immediate toxicity. Although an inverse linear relationship between the size of carriers and per cent efficacy in reduction of spleen parasite load was established, involvement of other factors such as drug release profiles or rates remains an open question. Because of greater efficacy and lesser toxicity, liposomal, niosomal and possibly microsphere-incorporated 14-deoxy-11-oxo-andrographolide might have clinical application to combat visceral Leishmaniasis.


