ClimbazoleCAS# 38083-17-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 38083-17-9 | SDF | Download SDF |
| PubChem ID | 37907.0 | Appearance | Powder |
| Formula | C15H17ClN2O2 | M.Wt | 292.76 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
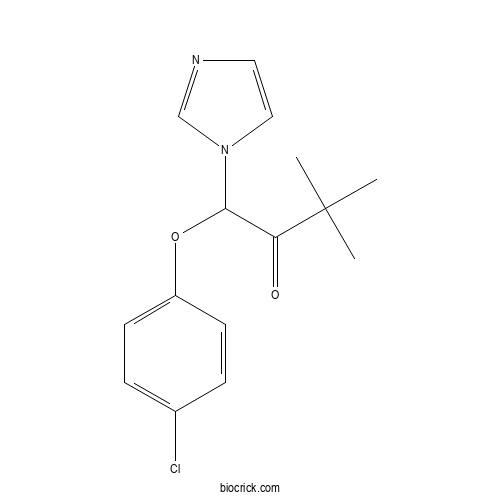
| Chemical Name | 1-(4-chlorophenoxy)-1-imidazol-1-yl-3,3-dimethylbutan-2-one | ||
| SMILES | CC(C)(C)C(=O)C(N1C=CN=C1)OC2=CC=C(C=C2)Cl | ||
| Standard InChIKey | OWEGWHBOCFMBLP-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C15H17ClN2O2/c1-15(2,3)13(19)14(18-9-8-17-10-18)20-12-6-4-11(16)5-7-12/h4-10,14H,1-3H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Climbazole Dilution Calculator

Climbazole Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.4158 mL | 17.0788 mL | 34.1577 mL | 68.3153 mL | 85.3942 mL |
| 5 mM | 0.6832 mL | 3.4158 mL | 6.8315 mL | 13.6631 mL | 17.0788 mL |
| 10 mM | 0.3416 mL | 1.7079 mL | 3.4158 mL | 6.8315 mL | 8.5394 mL |
| 50 mM | 0.0683 mL | 0.3416 mL | 0.6832 mL | 1.3663 mL | 1.7079 mL |
| 100 mM | 0.0342 mL | 0.1708 mL | 0.3416 mL | 0.6832 mL | 0.8539 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Oxysophoridine
Catalog No.:BCX0913
CAS No.:54809-74-4
- Graniline
Catalog No.:BCX0912
CAS No.:40737-97-1
- Dehydrocostuslactone
Catalog No.:BCX0911
CAS No.:74299-48-2
- Sodiumnewhouttuyfonate
Catalog No.:BCX0910
CAS No.:83766-73-8
- Isomaltotriose
Catalog No.:BCX0909
CAS No.:3371-50-4
- Fructo-oligosaccharideDP8/GF7
Catalog No.:BCX0908
CAS No.:62512-21-4
- 1-Kestoheptaose
Catalog No.:BCX0907
CAS No.:62512-20-3
- Maltooctaose
Catalog No.:BCX0906
CAS No.:6156-84-9
- 5-Hydroxytryptophan
Catalog No.:BCX0905
CAS No.:114-03-4
- 8-Methoxyquinoline-2-carboxylicacid
Catalog No.:BCX0904
CAS No.:21141-35-5
- Sodium taurolithocholate
Catalog No.:BCX0903
CAS No.:6042-32-6
- TaurohyodeoxycholicAcid
Catalog No.:BCX0902
CAS No.:2958-04-5
- Chlorine6
Catalog No.:BCX0915
CAS No.:19660-77-6
- PALATINOSE
Catalog No.:BCX0916
CAS No.:13718-94-0
- 8-Methoxyquinoline-2-carbaldehyde
Catalog No.:BCX0917
CAS No.:103854-64-4
- Cinnamylacetate
Catalog No.:BCX0918
CAS No.:103-54-8
- 4-Dimethylaminobenzaldehyde
Catalog No.:BCX0919
CAS No.:100-10-7
- SalicylaldehydeAzine
Catalog No.:BCX0920
CAS No.:959-36-4
- VanillylButylEther
Catalog No.:BCX0921
CAS No.:82654-98-6
- D-Pyroglutamicacid
Catalog No.:BCX0922
CAS No.:4042-36-8
- BoeravinoneA
Catalog No.:BCX0923
CAS No.:114567-33-8
- Pseudojervine
Catalog No.:BCX0924
CAS No.:36069-05-3
- MogrosideIIA
Catalog No.:BCX0925
CAS No.:1613527-65-3
- 14-Deoxy-11-oxoandrographolide
Catalog No.:BCX0926
CAS No.:42895-57-8
Evaluation of novel cosmetic shampoo formulations against Malassezia species: Preliminary results of anti-dandruff shampoo formulations.[Pubmed:38544350]
J Cosmet Dermatol. 2024 Mar 27.
OBJECTIVES: Malassezia species are common, clinically relevant, and lipid-dependent yeasts of humans. They are also the leading causes of the dandruff problem of humans, and the azoles are used primarily in their topical and systemic treatment. Resistance to azoles is an emerging problem among Malassezia sp., which indicates the need of new drug assessments that will be effective against dandruff and limit the use of azoles and other agents in treatment. Among them, the efficacy of various combinations of piroctone olamine and Climbazole against Malassezia sp. is highly important. Here, we assessed the efficacies of various piroctone olamine and Climbazole formulations against Malassezia sp. in comparison with ketoconazole. METHODS: A total of nine formulations were included in the study, where each formulation was prepared from different concentrations of piroctone olamine and Climbazole and both. All formulations contained the same ingredients as water, surfactants, hair conditioning agents, and preservatives. Malassezia furfur CBS1878, Malassezia globosa CBS7874, and Malassezia sympodialis CBS9570 were tested for antifungal susceptibility of each formulation by agar diffusion method. Sizes of the inhibition zones were compared with standard medical shampoo containing 2% ketoconazole, and the data were analyzed by Dunnett's multiple-comparison test. RESULTS: For all Malassezia sp. strains, Climbazole 0.5% and piroctone olamine/Climbazole (0.1%/0.1% and 0.1%/0.5%) combinations were found to have the same effect as the medical shampoo containing 2% ketoconazole. Piroctone olamine/Climbazole 1.0%/0.1% formulation showed the same efficacy as 2% ketoconazole on M. furfur and M. sympodialis, while 0.1%/0.5% formulation to only M. furfur. For M. globosa, none of the formulations tested were as effective as ketoconazole. CONCLUSION: The species distribution of Malassezia sp. varies depending on the anatomical location on the host. According to the results of this study, Climbazole and piroctone olamine combinations seem to be promising options against the dandruff problem with their high antifungal/anti dandruff efficacy.
The azole biocide climbazole induces oxidative stress, inflammation, and apoptosis in fish gut.[Pubmed:38453063]
Sci Total Environ. 2024 May 1;923:171475.
Climbazole is an azole biocide that has been widely used in formulations of personal care products. Climbazole can cause developmental toxicity and endocrine disruption as well as gut disturbance in aquatic organisms. However, the mechanisms behind gut toxicity induced by Climbazole still remain largely unclear in fish. Here, we evaluate the gut effects by exposing grass carp (Ctenopharyngodon idella) to Climbazole at levels ranging from 0.2 to 20 mug/L for 42 days by evaluating gene transcription and expression, biochemical analyses, correlation network analysis, and molecular docking. Results showed that Climbazole exposure increased cyp1a mRNA expression and ROS level in the three treatment groups. Climbazole also inhibited Nrf2 and Keap1 transcripts as well as proteins, and suppressed the transcript levels of their subordinate antioxidant molecules (cat, sod, and ho-1), increasing oxidative stress. Additionally, Climbazole enhanced NF-kappaB and ikappaBalpha transcripts and proteins, and the transcripts of NF-kappaB downstream pro-inflammatory factors (tnfalpha, and il-1beta/6/8), leading to inflammation. Climbazole increased pro-apoptosis-related genes (fadd, bad1, and caspase3), and decreased anti-apoptosis-associated genes (bcl2, and bcl-xl), suggesting a direct reaction to apoptosis. The molecular docking data showed that Climbazole could form stable hydrogen bonds with CYP1A. Mechanistically, our findings suggested that Climbazole can induce inflammation and oxidative stress through CYP450s/ROS/Nrf2/NF-kappaB pathways, resulting in cell apoptosis in the gut of grass carp.
Human adenovirus type 3 restores pharmacologically inhibited exosomal cargo in lung carcinoma cells.[Pubmed:38449802]
Front Pharmacol. 2024 Feb 21;15:1339862.
Introduction: Drug repurposing is fast growing and becoming an attractive approach for identifying novel targets, such as exosomes for cancer and antiviral therapy. Exosomes are a specialized class of extracellular vesicles that serve as functional mediators in intercellular communication and signaling that are important in normal physiological functions. A continuously growing body of evidence has established a correlation between the abnormal release of exosomes with various viral disease pathologies including cancer. Cells that are virus-infected release exosomes known to influence the process via the loading and transfer of viral components, such as miRNA, small (s) RNA, DNA, and proteins. Inhibition of exosome release may abate the spread and severity of viral infection, thus making exosomes an attractive target for antiviral therapies. We previously demonstrated the pharmacological inhibition of exosomes. Methods: Herein, we used a cell-based assay to determine the effect of Human adenovirus type 3 (HAdV3) on the exosome inhibition process by azole and Heparin derivatives. HAdV3-infected cells were treated with two concentrations of each inhibitor at different time points. Results: HAdV3 activities led to increased total sRNA, DNA, and exosome particle concentrations via particle tracking in the presence of Climbazole and Heparin relative to uninfected exosomes. In addition, there was an increased expression of classical markers such as ALG-2 interacting protein X (ALIX), and tetraspanin (CD63), (p < 0.05) and upregulated transcription factor interferon regulatory factor (IRF) 8 in the presence of HAdV3 after 24 hours (h) of treatment. Whereas higher concentrations of Climbazole and Heparin sodium salt were found to inhibit total exosome protein (p < 0.001) and exo-RNA (p < 0.01) content even in the presence of HAdV3 relative to infected exosomes only. Activities of HAdV3 in the presence of selected inhibitors resulted in the positive regulation of exosome related DNA damage/repair signaling proteins. Blocking exosome secretion partially obstructed viral entry. Immunological studies revealed that HAdV3 fiber protein levels in A549 cells were reduced at all concentrations of Climbazole and Heparin and both multiplicities of infections (p < 0.001). Discussion: Our findings suggest that while HAdV may bolster inhibited exosome content and release when modulating certain activities of the endosomal pathway mediators, HAdV entry might be constrained by the activities of these pharmacological agents.
Occurrence and distribution of azole antifungal agents in eight urban Romanian waste water treatment plants.[Pubmed:38369155]
Sci Total Environ. 2024 Apr 10;920:170898.
Azole compounds are utilized to combat fungal infections in plants to protect them and also used for treating mycosis in humans. The LC-MS/MS method is a technique that combines liquid chromatography with tandem mass spectrometry for analysis of twelve azole compounds from wastewater (influent, effluent) and sewage sludge. The compounds were isolated from waste water using automatic extraction in the solid phase. Sludge samples were dried by lyophilization, after which they were subjected to ultrasound extraction with methanol. The quantification limits ranged from 0.3 ng/L (clotrimazole-CLO and prochloraz-PRO) to 1.5 ng/L (tetraconazole-TEB and penconazole-PEN), for wastewater samples and for sewage sludge, the LOQs ranged from 0.1 ng/g to 0.6 ng/g. High concentrations of Climbazole-CLI (207-391 ng/L), tebuconazole (92-424 ng/L), and clotrimazole (6.9-93-ng/L) were observed in influent samples of the 8 urban wastewater treatment plants, followed by fluconazole (49.3-76.8 ng/L), and prochloraz (7.3-72 ng/L). The summation operatorAzoles had a maximum of 676 ng/L in the Galati effluent, followed by the Bucharest station 357 ng/L, and 345 ng/L in the Braila effluent. The highest value of the daily mass loading (input) level was observed for Climbazole, 265 mg/day/1000 in Iasi station, followed by tebuconazole, 238 mg/day/1000 people in the Bucharest station, and 203 mg/day/1000 people for Climbazole in the Targoviste station. The daily mass emission presented values between 0.7 and 247 mg/day/1000 people. The highest emissions were observed for Climbazole, 247 mg/day/1000 people in Braila station; 174 mg/day/1000 people in the Iasi station and 129 mg/day/1000 people in the Bucharest station. The concentrations of Climbazole detected in the effluent can present a high risk for the plants Lemna minor and Navicula pelliculosa. Clotrimazole may present a high risk to the plant Desmodesmus subspicatus and to the invertebrate Daphnia magna. PRO may present high risk to the invertebrate Mysidopsis Bahia.
Rational development of topical climbazole formulations.[Pubmed:38331330]
Int J Pharm. 2024 Mar 25;653:123886.
Dandruff, or pityriasis capitis simplex, is a common scalp condition associated with excessive flaking and scaling of the epidermal tissue. Other features include irregular corneocyte turnover, irritation, itching and an impaired skin barrier function. Previously we reported the characterization of Climbazole (CBZ), an antifungal agent used in the management of dandruff. Skin permeation of CBZ from neat solvents was also investigated. In the present work we evaluated CBZ permeation in human skin in vitro from more complex formulations that better represent products used by consumers. The various systems studied were composed of propylene glycol (PG), Transcutol(R)P (TC), octyl salicylate (OSal) and isopropyl alcohol (IPA). As well as measurement of skin uptake and penetration of CBZ, where possible, the skin retention and permeation of the various solvents was also determined. All vehicles promoted skin permeation of CBZ but no significant differences in amount permeated were evident between the binary vehicles (PG:TC, TC:OSal) and the ternary vehicle studied (PG:IPA:OSal). The binary vehicles generally promoted more skin uptake of CBZ compared with the neat solvents (PG, TC, OSal) studied previously. Permeation and skin extraction of CBZ from the PG:TC vehicles increased with increasing PG content; a similar trend was evident for the PG:IPA:OSal systems. New methods were developed and validated for measurement of PG, TC and OSal. Analysis of the individual solvents indicated that PG permeation was also independent of the amounts of other solvents in the binary or ternary systems. Consistent with previous findings higher proportions of TC permeated compared with PG for the PG:TC binary systems; TC also permeated the skin more rapidly than PG from these vehicles. For OSal, skin extraction was generally higher for TC:OSal compared with the PG:IPA:OSal vehicle. However, increasing the content of OSal did not appear to influence CBZ skin uptake nor permeation. Interestingly, the effects of the various PG:TC vehicles on CBZ skin delivery contrast with results we previous reported for the same systems for a different active. This confirms that with reference to skin permeation, formulation effects and/or skin penetration enhancement should be expected to vary and may not be predicted for specific vehicles.
An azole fungicide climbazole damages the gut-brain axis in the grass carp.[Pubmed:38219582]
J Hazard Mater. 2024 Mar 5;465:133463.
Azole antifungal Climbazole has frequently been detected in aquatic environments and shows various effects in fish. However, the underlying mechanism of toxicity through the gut-brain axis of Climbazole is unclear. Here, we investigated the effects of Climbazole at environmental concentrations on the microbiota-intestine-brain axis in grass carp via histopathological observation, gene expression and biochemical analyses, and high-throughput sequencing of the 16 S rRNA. Results showed that exposure to 0.2 to 20 mug/L Climbazole for 42 days significantly disrupted gut microbiota and caused brain neurotoxicity in grass carp. In this study, there was an alteration in the phylum and genus compositions in the gut microbiota following Climbazole treatment, including reducing Fusobacteria (e.g., Cetobacterium) and increasing Actinobacteria (e.g., Nocardia). Climbazole disrupted intestinal microbial abundance, leading to increased levels of lipopolysaccharide and tumor necrosis factor-alpha in the gut, serum, and brain. They passed through the impaired intestinal barrier into the circulation and caused the destruction of the blood-brain barrier through the gut-brain axis, allowing them into the brain. In the brain, Climbazole activated the nuclear factor kappaB pathway to increase inflammation, and suppressed the E2-related factor 2 pathway to produce oxidative damage, resulting in apoptosis, which promoted neuroinflammation and neuronal death. Besides, our results suggested that this neurotoxicity was caused by the breakdown of the microbiota-gut-brain axis, mediated by reduced concentrations of dopamine, short chain fatty acids, and intestinal microbial activity induced by Climbazole.
Climbazole causes cell apoptosis and lipidosis in the liver of grass carp.[Pubmed:37722153]
Aquat Toxicol. 2023 Oct;263:106698.
Climbazole, an azole, is widely used in personal care products, pharmaceuticals, and pesticides and is frequently detected in surface water. Climbazole has showed endocrine-disrupting effects. However, the effects of Climbazole in fish are still largely unclear. In this study, grass carp (Ctenopharyngodon idella) and liver cell lines (L8824 cells) were treated with Climbazole at concentrations ranging from 0.2 to 20 mug/L for 42 days in vivo and 24 h in vitro to evaluate the effects on the liver, respectively. Pathological, biochemical, and gene transcription and expression analyses were conducted to examine the hepatotoxicity. Our results showed that Climbazole significantly decreased the hepatosomatic index, caused cell apoptosis in vivo and in vitro, and finally accumulated lipids in the liver. Beside, Climbazole increased ROS levels, reduced Nrf2 and Keap1 mRNA and protein levels, and further decreased transcription of Nrf2-dependent downstream antioxidant enzyme genes, causing oxidative stress. Moreover, Climbazole increased transcription and protein levels of apoptosis-related genes. Finally, Climbazole damaged mitochondrial function and structure, disrupted liver lipid metabolism. Overall, Climbazole caused hepatotoxicity, leading to a high ecological risk for aquatic organisms.
Uptake and accumulation of emerging contaminants in processing tomato irrigated with tertiary treated wastewater effluent: a pilot-scale study.[Pubmed:37692419]
Front Plant Sci. 2023 Aug 23;14:1238163.
The reuse of treated wastewater for crop irrigation is vital in water-scarce semi-arid regions. However, concerns arise regarding emerging contaminants (ECs) that persist in treated wastewater and may accumulate in irrigated crops, potentially entering the food chain and the environment. This pilot-scale study conducted in southern Italy focused on tomato plants (Solanum lycopersicum L. cv Taylor F1) irrigated with treated wastewater to investigate EC uptake, accumulation, and translocation processes. The experiment spanned from June to September 2021 and involved three irrigation strategies: conventional water (FW), treated wastewater spiked with 10 target contaminants at the European average dose (TWWx1), and tertiary WWTP effluent spiked with the target contaminants at a triple dose (TWWx3). The results showed distinct behavior and distribution of ECs between the TWWx1 and TWWx3 strategies. In the TWWx3 strategy, clarithromycin, carbamazepine, metoprolol, fluconazole, and Climbazole exhibited interactions with the soil-plant system, with varying degradation rates, soil accumulation rates, and plant accumulation rates. In contrast, naproxen, ketoprofen, diclofenac, sulfamethoxazole, and trimethoprim showed degradation. These findings imply that some ECs may be actively taken up by plants, potentially introducing them into the food chain and raising concerns for humans and the environment.
Development of a simplified human embryonic stem cell-based retinal pre-organoid model for toxicity evaluations of common pollutants.[Pubmed:37602871]
Cutan Ocul Toxicol. 2023 Dec;42(4):264-272.
OBJECTIVE: To explore the retinal toxicity of pharmaceuticals and personal care products (PPCPs), flame retardants, bisphenols, phthalates, and polycyclic aromatic hydrocarbons (PAHs) on human retinal progenitor cells (RPCs) and retinal pigment epithelial (RPE) cells, which are the primary cell types at the early stages of retinal development, vital for subsequent functional cell type differentiation, and closely related to retinal diseases. MATERIALS AND METHODS: After 23 days of differentiation, human embryonic stem cell (hESC)-based retinal pre-organoids, containing RPCs and RPE cells, were exposed to 10, 100, and 1000 nM pesticides (butachlor, terbutryn, imidacloprid, deltamethrin, pendimethalin, and carbaryl), flame retardants (PFOS, TBBPA, DBDPE, and TDCIPP), PPCPs (Climbazole and BHT), and other typical pollutants (phenanthrene, DCHP, and BPA) for seven days. Then, mRNA expression changes were monitored and compared. RESULTS: (1) The selected pollutants did not show strong effects at environmental and human-relevant concentrations, although the effects of flame retardants were more potent than those of other categories of chemicals. Surprisingly, some pollutants with distinct structures showed similar adverse effects. (2) Exposure to pollutants induced different degrees of cell detachment, probably due to alterations in extracellular matrix and/or cell adhesion. CONCLUSIONS: In this study, we established a retinal pre-organoid model suitable for evaluating multiple pollutants' effects, and pointed out the potential retinal toxicity of flame retardants, among other pollutants. Nevertheless, the potential mechanisms of toxicity and the effects on cell detachment are still unclear and deserve further exploration. Additionally, this model holds promise for screening interventions aimed at mitigating the detrimental effects of these pollutants.
Evaluation of residual antibacterial effects on canine skin surface and hair following treatment with five commercial mousse products against Staphylococcus pseudintermedius.[Pubmed:37434336]
Vet Dermatol. 2023 Dec;34(6):495-504.
BACKGROUND: Antibacterial effect studies of commercial antiseptics typically have evaluated hair and not the skin. OBJECTIVES: To evaluate the antibacterial effects of mousse products on both canine skin and hair. ANIMALS: Fifteen short-haired and eight long-haired dogs without skin disease. MATERIALS AND METHODS: Five mousses were applied once: (1) 2% chlorhexidine and 2% miconazole; (2) 0.05% phytosphingosine; (3) 2% salicylic acid and 10% ethyl lactate; (4) 3% chlorhexidine and 0.5% Climbazole; and (5) 2% chlorhexidine and 1% ketoconazole. Skin swabs and hair were collected from application sites before treatment, and at 1 h and at Day (D)2, D4, D8, D10 and D14 post-treatment. Skin swabs and hair were placed on Mueller-Hinton plates inoculated with Staphylococcus pseudintermedius inoculum suspension. Inhibition zones were measured after incubation. RESULTS: Inhibition was not noted with mousses 2 and 3. In mousse 5, inhibition zone sizes produced by swabs from long- and short-haired dogs were not significantly different (p = 0.105), and all swabs and hair produced inhibition until D14, regardless of hair length. By contrast, in mousse 1, inhibition zones produced by swabs from long-haired dogs were smaller than those from short-haired dogs (p < 0.001), and swabs from long-haired dogs produced a shorter duration of bacterial inhibition than hair. CONCLUSIONS AND CLINICAL RELEVANCE: The antibacterial effects of mousse 5 were not affected by hair length. Hair may be acceptable for evaluating effects on the skin in short-haired dogs. However, long hair may interfere with product distribution and duration of bacterial inhibition. Therefore, the evaluation of hair alone may overestimate clinically relevant antibacterial effects.
Does climbazole instigate a threat in the environment as persistent, mobile and toxic compound? Unveiling the occurrence and potential ecological risks of its phototransformation products in the water cycle.[Pubmed:37354716]
J Hazard Mater. 2023 Sep 15;458:131854.
Persistent, mobile, and toxic chemicals (PMT), such as the antimycotic Climbazole-(CBZ), proliferate in water cycle and imperil drinking water quality, sparking off research about their environmental fate. Unlike the parent compound, its transformation products-(TPs) are scarcely investigated, much less as PMTs. To this end, phototransformation of CBZ was investigated. A novel suspect-screening workflow was developed and optimized by cross-comparing the results of the identified photo-TPs against literature data to create an enhanced HRMS-database for environmental investigations of CBZ/TPs in the water cycle. In total, 24 TPs were identified, 14 of which are reported for the first time. Isomerism, dechlorination, hydroxylation, and cleavage of the ether or C-N bond are suggested as the main transformation routes. A screening of CBZ/TPs was conducted in wastewater, leachates, surface, and groundwater, revealing a maximum concentration of 464.8 ng/L in groundwater. In silico and in vitro methods were used for toxicity assessment, indicating toxicity for CBZ and some TPs. Seemingly, CBZ is rightly considered as PMT, and a higher potential to occur in surface or groundwater than non-PM chemicals appears. Likewise, the occurrence of TPs due to PMT properties or emission patterns was evaluated.
Selective pharmacological inhibition alters human carcinoma lung cell-derived extracellular vesicle formation.[Pubmed:37303541]
Heliyon. 2023 May 30;9(6):e16655.
Exosomes also termed small extracellular vesicles (sEVs) are important mediators of intercellular communication in many physiological and pathological processes such as protein clearance, immunity, infections, signaling, and cancer. Elevated circulating levels of exosomes have been linked to some viral infections, aggressive cancer, and neurodegenerative diseases. Some pharmacological compounds have been demonstrated to effectively inhibit exosome production pathways. There are very few studies on exosome inhibition and how they influence pathophysiological conditions. METHODS: In the current study, we examined how inhibition of extracellular vesicle release and/or uptake would impact the exosome formation pathway. Using a constellation of improved EV experimental approaches, we evaluated the concentration-based cytotoxicity effects of pharmacological agents (ketoconazole, Climbazole, and heparin) on Human Lung Carcinoma (A549) cell viability. We investigated the effect of inhibitor dosages on exosome production and release. Analysis of exosome inhibition includes quantitative analysis and total protein expression of exosome release after pharmacological inhibition; we examined exosome protein level after inhibition. RESULTS: Selective inhibition of exosomes altered particle sizes, and heparin significantly reduced the total exosomes released. Climbazole and heparin undermined membrane-bound tetraspanin CD63 expression and significantly disrupted ALIX protein (p Climbazole and heparin as effective inhibitors of exosome synthesis.
Prioritization of pharmaceuticals and personal care products in the surface waters of Korea: Application of an optimized risk-based methods.[Pubmed:37201424]
Ecotoxicol Environ Saf. 2023 Jul 1;259:115024.
The occurrence of PPCPs in aquatic environments and their potential adverse effects on aquatic organisms have raised worldwide concerns. To address this issue, a study was conducted to analyze 137 selected PPCPs in Korean surface waters, and an optimized risk-based prioritization was performed. The results revealed that 120 PPCPs were detected, with 98 quantified at concentrations ranging from few ng/L to 42,733 ng/L for metformin. The 95% upper confidence limit (UCL95) of the mean value of the measured environmental concentration (MEC) for Metformin was about eight times higher than the second highest compound, dimethyl phthalate, indicating that antidiabetic groups had the highest concentration among the therapeutic groups. An optimized risk-based prioritization was then assessed based on the multiplication of two indicators, the Frequency of Exceedance and the Extent of Exceedance of Predicted No-Effect Concentrations (PNECs), which can be calculated using the traditional risk quotient (RQ) approach. The study found that clotrimazole had the highest risk quotient value of 17.4, indicating a high risk to aquatic organisms, with seven and 13 compounds showing RQ values above 1 and 0.1, respectively. After considering the frequency of exceedance, clotrimazole still had the highest novel risk quotient (RQ(f)) value of 17.4, with 99.6% of its MECs exceeding PNECs. However, the number of compounds with RQ(f) values above 1 decreased from seven to five, with cetirizine and flubendazole being excluded. Furthermore, only 10 compounds exhibited RQ(f) values above 0.1. The study also observed significant differences in the results between risk-based and exposure-based prioritization methods, with only five compounds, cetirizine, olmesartan, Climbazole, sulfapyridine, and imidacloprid, identified in both methods. This finding highlights the importance of considering multiple methods for prioritizing chemicals, as different approaches may yield different results.
Managing PMT/vPvM substances in consumer products through the concepts of essential-use and functional substitution: a case-study for cosmetics.[Pubmed:37199459]
Environ Sci Process Impacts. 2023 Jun 21;25(6):1067-1081.
Measures are needed to protect water sources from substances that are mobile, persistent and toxic (PMT) or very persistent and very mobile (vPvM). PMT/vPvM substances are used in a diverse range of applications, including consumer products. The combined application of the essential-use and functional substitution concepts has been proposed to phase out substances of concern and support the transition to safer and more sustainable chemicals, a key goal of the European Commission's Chemicals Strategy for Sustainability. Here, we first identified the market share of PMT/vPvM containing cosmetic products. We found that 6.4% of cosmetic products available on the European market contain PMT or vPvM substances. PMT/vPvM substances were most often found in hair care products. Based on their high occurrence, the substances Allura red (CAS 25956-17-6), benzophenone-4 (CAS 4065-45-6) and Climbazole (CAS 38083-17-9) were selected as case-studies for assessment of their functionality, availability of safer alternatives and essentiality. Following the functional substitution framework, we found that the technical function of Allura red was not necessary for the performance of some cosmetic products, making the use non-essential. For other applications of Allura red, as well as all applications of benzophenone-4 and Climbazole, the technical function of the chemical was considered necessary for the performance. Via the alternative's assessment procedure, which used experimental and in silico data and three different multicriteria decision analysis (MCDA) strategies, safer alternatives were identified for all case-study chemicals. All assessed uses of PMT/vPvM substances were thus deemed non-essential and should consequently be phased out.
Toxicity interactions of azole fungicide mixtures on Chlorella pyrenoidosa.[Pubmed:36947457]
Environ Toxicol. 2023 Jul;38(7):1509-1519.
It is acknowledged that azole fungicides may release into the environment and pose potential toxic risks. The combined toxicity interactions of azole fungicide mixtures, however, are still not fully understood. The combined toxicities and its toxic interactions of 225 binary mixtures and 126 multi-component mixtures on Chlorella pyrenoidosa were performed in this study. The results demonstrated that the negative logarithm 50% effect concentration (pEC(50) ) of 10 azole fungicides to Chlorella pyrenoidosa at 96 h ranged from 4.23 (triadimefon) to 7.22 (ketoconazole), while the pEC(50) values of the 351 mixtures ranged from 3.91 to 7.44. The high toxicities were found for the mixtures containing epoxiconazole. According to the results of the model deviation ratio (MDR) calculated from the concentration addition (MDR(CA) ), 243 out of 351 (69.23%) mixtures presented additive effect at the 10% effect, while the 23.08% and 7.69% of mixtures presented synergistic and antagonistic effects, respectively. At the 30% effect, 47.29%, 29.34%, and 23.36% of mixtures presented additive effects, synergism, and antagonism, respectively. At the 50% effect, 44.16%, 34.76%, and 21.08% of mixtures presented additive effects, synergism, and antagonism, respectively. Thus, the toxicity interactions at low concentration (10% effect) were dominated by additive effect (69.23%), whereas 55.84% of mixtures induced synergism and antagonism at high concentration (50% effect). Climbazole and imazalil were the most frequency of components presented in the additive mixtures. Epoxiconazole was the key component induced the synergistic effects, while clotrimazole was the key component in the antagonistic mixtures.


