IsopedicinCAS# 4431-42-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 4431-42-9 | SDF | Download SDF |
| PubChem ID | 102004576 | Appearance | Cryst. |
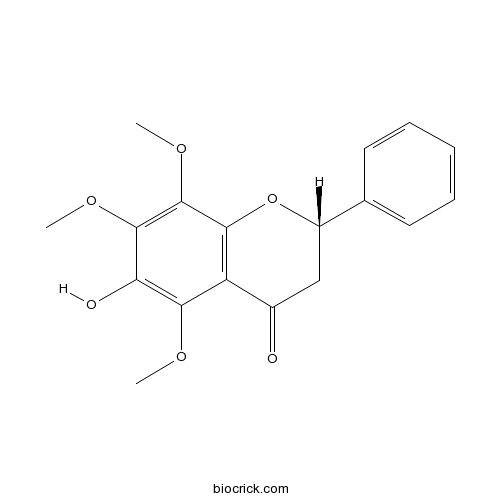
| Formula | C18H18O6 | M.Wt | 330.34 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (2S)-6-hydroxy-5,7,8-trimethoxy-2-phenyl-2,3-dihydrochromen-4-one | ||
| SMILES | COC1=C(C(=C(C2=C1C(=O)CC(O2)C3=CC=CC=C3)OC)OC)O | ||
| Standard InChIKey | CSVYPJWNGKLMJM-LBPRGKRZSA-N | ||
| Standard InChI | InChI=1S/C18H18O6/c1-21-15-13-11(19)9-12(10-7-5-4-6-8-10)24-16(13)18(23-3)17(22-2)14(15)20/h4-8,12,20H,9H2,1-3H3/t12-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Isopedicin has anti-inflammatory functions, it inhibits the O(2)(*)(-) production in human neutrophils by an elevation of cellular cAMP and activation of PKA through its inhibition of cAMP-specific PDE. |
| Targets | PKA | cAMP | PDE | p38MAPK | NADPH-oxidase | JNK |

Isopedicin Dilution Calculator

Isopedicin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.0272 mL | 15.1359 mL | 30.2718 mL | 60.5437 mL | 75.6796 mL |
| 5 mM | 0.6054 mL | 3.0272 mL | 6.0544 mL | 12.1087 mL | 15.1359 mL |
| 10 mM | 0.3027 mL | 1.5136 mL | 3.0272 mL | 6.0544 mL | 7.568 mL |
| 50 mM | 0.0605 mL | 0.3027 mL | 0.6054 mL | 1.2109 mL | 1.5136 mL |
| 100 mM | 0.0303 mL | 0.1514 mL | 0.3027 mL | 0.6054 mL | 0.7568 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Ligustilide
Catalog No.:BCN1031
CAS No.:4431-01-0
- Metronidazole
Catalog No.:BCC9046
CAS No.:443-48-1
- Tabersonine
Catalog No.:BCN5496
CAS No.:4429-63-4
- Methyl isodrimeninol
Catalog No.:BCN5495
CAS No.:442851-27-6
- Anguizole
Catalog No.:BCC1365
CAS No.:442666-98-0
- JW 67
Catalog No.:BCC6251
CAS No.:442644-28-2
- CK 666
Catalog No.:BCC6088
CAS No.:442633-00-3
- CK-636
Catalog No.:BCC5107
CAS No.:442632-72-6
- Aspertine C
Catalog No.:BCN2153
CAS No.:442155-62-6
- Clemizole
Catalog No.:BCC1485
CAS No.:442-52-4
- Harmine
Catalog No.:BCN5494
CAS No.:442-51-3
- Macitentan
Catalog No.:BCC1142
CAS No.:441798-33-0
- SF 11
Catalog No.:BCC7805
CAS No.:443292-81-7
- Z-DL-Met-OH
Catalog No.:BCC2759
CAS No.:4434-61-1
- BC 11 hydrobromide
Catalog No.:BCC2381
CAS No.:443776-49-6
- JNJ-7706621
Catalog No.:BCC2171
CAS No.:443797-96-4
- 3-Chloro-4-(3-fluorobenzyloxy)nitrobenzene
Catalog No.:BCC8626
CAS No.:443882-99-3
- Norkhellol
Catalog No.:BCN5497
CAS No.:4439-68-3
- Glycosmisic acid
Catalog No.:BCN3650
CAS No.:443908-19-8
- Vandetanib (ZD6474)
Catalog No.:BCC3883
CAS No.:443913-73-3
- 1,4-Benzodioxane-6-carboxylic acid
Catalog No.:BCC8422
CAS No.:4442-54-0
- 2-Cyclohexylethylamine
Catalog No.:BCN1794
CAS No.:4442-85-7
- Norwogonin
Catalog No.:BCN3190
CAS No.:4443-09-8
- Pazopanib (GW-786034)
Catalog No.:BCC1286
CAS No.:444731-52-6
Potent inhibition of superoxide anion production in activated human neutrophils by isopedicin, a bioactive component of the Chinese medicinal herb Fissistigma oldhamii.[Pubmed:19100830]
Free Radic Biol Med. 2009 Feb 15;46(4):520-8.
Fissistigma oldhamii is widely used in traditional Chinese medicine to treat rheumatoid arthritis. Activation of neutrophils is a key feature of inflammatory diseases. Herein, the anti-inflammatory functions of Isopedicin, a flavanone derived from F. oldhamii, and its underlying mechanisms were investigated in human neutrophils. Isopedicin potently and concentration-dependently inhibited superoxide anion (O(2)(*)(-)) production in formyl-L-methionyl-L-leucyl-L-phenylalanine (FMLP)-activated human neutrophils with an IC(50) value of 0.34+/-0.03 microM. Furthermore, Isopedicin displayed no superoxide-scavenging ability, and it failed to alter subcellular NADPH oxidase activity. The inhibitory effect of Isopedicin on O(2)(*)(-) production was reversed by protein kinase A (PKA) inhibitors. Moreover, Isopedicin increased cAMP formation and PKA activity in FMLP-activated human neutrophils, which occurred through the inhibition of phosphodiesterase (PDE) activity but not an increase in adenylate cyclase function. In addition, Isopedicin reduced FMLP-induced phosphorylation of extracellular regulated kinase and c-Jun N-terminal kinase, which was reversed by the PKA inhibitor. In contrast, Isopedicin failed to alter FMLP-induced phosphorylation of p38 mitogen-activated protein kinase and calcium mobilization. In summary, these results demonstrate that inhibition of O(2)(*)(-) production in human neutrophils by Isopedicin is associated with an elevation of cellular cAMP and activation of PKA through its inhibition of cAMP-specific PDE.


