MacitentanEndothelin (ET)(A) and ET(B) receptor antagonist CAS# 441798-33-0 |
- Atrasentan
Catalog No.:BCC1379
CAS No.:173937-91-2
- sitaxsentan
Catalog No.:BCC1951
CAS No.:184036-34-8
- Zibotentan (ZD4054)
Catalog No.:BCC2524
CAS No.:186497-07-4
- Atrasentan hydrochloride
Catalog No.:BCC1380
CAS No.:195733-43-8
- Avosentan
Catalog No.:BCC1387
CAS No.:290815-26-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 441798-33-0 | SDF | Download SDF |
| PubChem ID | 16004692 | Appearance | Powder |
| Formula | C19H20Br2N6O4S | M.Wt | 588.27 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 50 mg/mL (84.99 mM) *"≥" means soluble, but saturation unknown. | ||
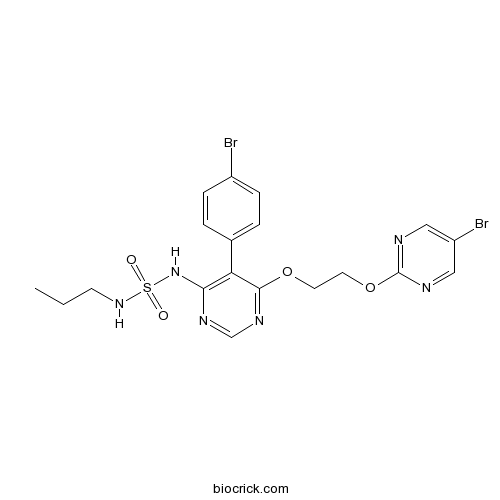
| Chemical Name | 5-(4-bromophenyl)-6-[2-(5-bromopyrimidin-2-yl)oxyethoxy]-N-(propylsulfamoyl)pyrimidin-4-amine | ||
| SMILES | CCCNS(=O)(=O)NC1=C(C(=NC=N1)OCCOC2=NC=C(C=N2)Br)C3=CC=C(C=C3)Br | ||
| Standard InChIKey | JGCMEBMXRHSZKX-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C19H20Br2N6O4S/c1-2-7-26-32(28,29)27-17-16(13-3-5-14(20)6-4-13)18(25-12-24-17)30-8-9-31-19-22-10-15(21)11-23-19/h3-6,10-12,26H,2,7-9H2,1H3,(H,24,25,27) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Macitentan is an orally active, non-peptide dual endothelin ETA and ETB receptor antagonist for the potential treatment of idiopathic pulmonary fibrosis (IPF) and pulmonary arterial hypertension (PAH). |

Macitentan Dilution Calculator

Macitentan Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.6999 mL | 8.4995 mL | 16.999 mL | 33.998 mL | 42.4975 mL |
| 5 mM | 0.34 mL | 1.6999 mL | 3.3998 mL | 6.7996 mL | 8.4995 mL |
| 10 mM | 0.17 mL | 0.8499 mL | 1.6999 mL | 3.3998 mL | 4.2497 mL |
| 50 mM | 0.034 mL | 0.17 mL | 0.34 mL | 0.68 mL | 0.8499 mL |
| 100 mM | 0.017 mL | 0.085 mL | 0.17 mL | 0.34 mL | 0.425 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Macitentan is a new dual ETA/ETB endothelin (ET) receptor antagonist, with mean IC50 values of 0.5 ± 0.2 nM (n= 17) to inhibit the binding of 125I-ET-1 to recombinant ETA receptors, and of 391±182 nM (n= 17) for ETB receptors in Chinese hamster ovary cells [1].
ETA and ETB are ET receptors, both of them mediate the detrimental actions of ET-1, and dual blockade of them may be necessary [1].
In microsomal membranes of human-ETA and ETB-overexpressing Chinese hamster ovary cells, macitentan inhibited the binding between 125I-ET-1 and recombinant ETA receptors, with a mean IC50 value of 0.5 ± 0.2 nM (n= 17). The mean IC50 value for ETB receptors was 391±182 nM (n= 17). Macitentan completely inhibited the effect that ET-1 increased intracellular calcium in non-recombinant cells [1].
Intravenous administrated macitentan had a volume of distribution largely exceeding plasma volume and a terminal half-life of 2 h in rats. Macitentan was hence metabolized to its major and the only circulating metabolite, a dual ET receptor antagonist, ACT-132577. ACT-132577 also had a volume of distribution greater than the plasma volume. It showed a longer half-life than macitentan in rats. In rat, multiple oral dosing of macitentan at a dose of 10 mg/kg led to 4 to 5-fold higher exposure levels of ACT-132577 than those of the parent compound [1].
Reference:
[1]. Marc Iglarz, Christoph Binkert, Keith Morrison, et al. Pharmacology of Macitentan, an Orally Active Tissue-Targeting Dual Endothelin Receptor Antagonist. Journal of Pharmacology and Experimental Therapeutics, 2008, 327:736-745.
- 6-Acetylacteoside
Catalog No.:BCC8109
CAS No.:441769-43-3
- 2-(Cyanomethyl)benzimidazole
Catalog No.:BCC8483
CAS No.:4414-88-4
- Eribulin mesylate
Catalog No.:BCC5173
CAS No.:441045-17-6
- Benzoin oxime
Catalog No.:BCC8858
CAS No.:441-38-3
- 3-Phenyl-2-propen-1-ol
Catalog No.:BCN5493
CAS No.:4407-36-7
- WAY 200070
Catalog No.:BCC7669
CAS No.:440122-66-7
- K 579
Catalog No.:BCC2364
CAS No.:440100-64-1
- Trifluoperazine 2HCl
Catalog No.:BCC4384
CAS No.:440-17-5
- Isoscabertopin
Catalog No.:BCN4634
CAS No.:439923-16-7
- Gnetifolin M
Catalog No.:BCN3394
CAS No.:439900-84-2
- NPY 5RA972
Catalog No.:BCC7747
CAS No.:439861-56-0
- BMS-509744
Catalog No.:BCC1424
CAS No.:439575-02-7
- Harmine
Catalog No.:BCN5494
CAS No.:442-51-3
- Clemizole
Catalog No.:BCC1485
CAS No.:442-52-4
- Aspertine C
Catalog No.:BCN2153
CAS No.:442155-62-6
- CK-636
Catalog No.:BCC5107
CAS No.:442632-72-6
- CK 666
Catalog No.:BCC6088
CAS No.:442633-00-3
- JW 67
Catalog No.:BCC6251
CAS No.:442644-28-2
- Anguizole
Catalog No.:BCC1365
CAS No.:442666-98-0
- Methyl isodrimeninol
Catalog No.:BCN5495
CAS No.:442851-27-6
- Tabersonine
Catalog No.:BCN5496
CAS No.:4429-63-4
- Metronidazole
Catalog No.:BCC9046
CAS No.:443-48-1
- Ligustilide
Catalog No.:BCN1031
CAS No.:4431-01-0
- Isopedicin
Catalog No.:BCN4633
CAS No.:4431-42-9
Early Experience of Macitentan for Pulmonary Arterial Hypertension in Adult Congenital Heart Disease.[Pubmed:28237536]
Heart Lung Circ. 2017 Oct;26(10):1113-1116.
BACKGROUND: Endothelin receptor antagonists (ERA) have been recognised as effective therapy for pulmonary arterial hypertension in congenital heart disease (CHD-PH), and Eisenmenger syndrome (ES) since The Bosentan Randomized Trial of Endothelin Antagonist Therapy-5 (Breathe 5) study. A new dual receptor antagonist - Macitentan - is currently undergoing trials to determine its efficacy in simple ES. To date there is little information on this therapy in CHD and we report our first experience, some with more complex diseases. METHODS: Data was collected prospectively from September 2014. Patients with CHD-PH were started on or converted to Macitentan if they required therapy with phosphodiesterase 5 inhibitor (PDE5i) or if there was insufficient response or a reaction to bosentan, especially those with trisomy 21. Patients were seen approximately three months after starting therapy to assess echocardiography, six minute walk test, clinical response and tolerability. All patients underwent monthly liver tests initially, but this was reduced to three-monthly in Q4 2015. RESULTS: Fifteen patients with CHD-PH (eight male, seven female) were started on Macitentan, median (range) age 38 (23-61) years, and eight patients with Down's syndrome. Eight patients had complex CHD with one having unoperated double inlet left ventricle with ventriculo-arterial discordance, one had double outlet right ventricle and six with complete atrio-ventricular septal defect. Six patients were ERA naive and nine patients changed from bosentan to Macitentan in order to achieve improved drug-drug interaction. Median length of time of treatment with Macitentan is 289 (0-694) days to date. One discontinued due to rash and feeling unwell; one was unable to comply with medication due to learning difficulties and one died soon after commencing rescue therapy. This last patient was functional class IV with oxygen saturation of 67% at rest, with right heart failure and was unable to perform a walk test before commencing therapy. All patients who remained on therapy had significant increase in six minute walk test from median 286 (120-426) to 360m (150-450)(p <0.05), most notably in those treatment naive. Functional class median remained at 3 but the range was reduced (1-3). Resting oxygen saturations improved from median 83 range (77-95%) at rest to 91 (77-96%) and at end walk from 78 (48-90%) to 79 (62-96%). Tricuspid regurgitant peak Doppler derived pressure drop did not change (as expected) at 4.6 (4.3-5.5)m/s. There were no episodes of liver dysfunction. CONCLUSIONS: The introduction of this new therapy has been simple and mostly well tolerated in our sick group of patients. With the usual reservations concerning the open-label nature of our observations, Macitentan has good signals regarding oxygen saturations and encouraging signals relating to efficacy.
Tolerability of Switch to Macitentan from Bosentan in Pulmonary Arterial Hypertension.[Pubmed:28257550]
South Med J. 2017 Mar;110(3):223-228.
OBJECTIVES: Pulmonary arterial hypertension (PAH) is a progressive disease that can be treated with several medications. Macitentan, an endothelin receptor antagonist (ERA), has received approval as a PAH therapy. We report our data regarding the tolerability in patients with PAH who were switched from bosentan to Macitentan. METHODS: At the Baylor Pulmonary Hypertension Program, 24 patients with PAH who had been taking bosentan and were switched to Macitentan were identified in this retrospective study. Data from these patients who switched from bosentan 125 mg orally twice per day to Macitentan 10 mg orally daily (between October 2013 and February 2015) when Macitentan became commercially available were collected. Patients were advised to take their last evening dose of bosentan and then take the first dose of Macitentan the following morning within 12 to 24 hours of the last bosentan dose. Baseline data and postswitch data, including 6-minute walk distance, brain naturietic peptide, alanine transaminase (ALT) and aspartate transaminase (AST) levels, World Health Organization Functional Class (WHO FC), Borg dyspnea score, presence of peripheral edema. RESULTS: At the time of the switch, the mean age was 58 +/- 13 (mean +/- standard deviation) years, the duration of disease was 6.6 +/- 4.4 years, 21 patients were women, 54% were white, and 25% had idiopathic PAH. The mean duration of follow-up after the switch was 5.7 +/- 1.5 months. The 6-minute walk distance was 344 +/- 106 m preswitch and 319 +/- 85 m postswitch (P = 0.18). Brain naturietic peptide levels were 91 +/- 170 pg/mL preswitch and 90 +/- 137 pg/mL postswitch (P = 0.93). At the time of the switch, 42% were WHO FC II and 50% had edema, and 55% had edema. AST and ALT remained unchanged postswitch. Two patients did not tolerate the switch to Macitentan and had to be returned to bosentan: one patient with portopulmonary hypertension developed elevated AST and ALT and the second patient's Macitentan was stopped because of malaise and tachyarrhythmia. One patient who underwent a successful liver transplant had Macitentan stopped following the transplant. CONCLUSIONS: A rapid switch from bosentan to Macitentan was well tolerated and safe with maintained WHO FC, with no significant change in edema and liver enzyme levels. The switch from bosentan to Macitentan eliminates the need for monthly liver function test monitoring and removes the potential for bosentan treatment interruption.
SERAPHIN haemodynamic substudy: the effect of the dual endothelin receptor antagonist macitentan on haemodynamic parameters and NT-proBNP levels and their association with disease progression in patients with pulmonary arterial hypertension.[Pubmed:28329315]
Eur Heart J. 2017 Apr 14;38(15):1147-1155.
Aims: The effect of Macitentan on haemodynamic parameters and NT-proBNP levels was evaluated in pulmonary arterial hypertension (PAH) patients in the SERAPHIN study. Association between these parameters and disease progression, assessed by the primary endpoint (time to first morbidity/mortality event), was explored. Methods and results: Of the 742 randomized patients, 187 with right heart catheterization at baseline and month 6 participated in a haemodynamic sub-study. Prespecified endpoints included change from baseline to month 6 in cardiac index (CI), right atrial pressure (RAP), mean pulmonary arterial pressure (mPAP), pulmonary vascular resistance (PVR), mixed-venous oxygen saturation, and NT-proBNP. Exploratory analyses examined associations between CI, RAP, and NT-proBNP and disease progression using the Kaplan-Meier method and Cox regression models. Macitentan improved CI, RAP, mPAP, PVR and NT-proBNP vs. placebo at month 6. Absolute levels of CI, RAP and NT-proBNP at baseline and month 6, but not their changes, were associated with morbidity/mortality events. Patients with CI > 2.5 L/min/m2, RAP < 8 mmHg, or NT-proBNP < 750 fmol/ml at month 6 had a lower risk of morbidity/mortality than those not meeting these thresholds (HR 0.49, 95% CL 0.28-0.86; HR 0.72, 95% CL 0.42-1.22; and HR 0.22, 95% CL 0.15-0.33, respectively). Conclusions: For all treatment groups, baseline and month 6 values of CI, RAP, and NT-proBNP, but not their changes, were associated with morbidity/mortality events, confirming their relevance in predicting disease progression in patients with PAH. By improving those parameters, Macitentan increased the likelihood of reaching threshold values associated with lower risk of morbidity/mortality.
Functional estimation of endothelin-1 receptor antagonism by bosentan, macitentan and ambrisentan in human pulmonary and radial arteries in vitro.[Pubmed:28300593]
Eur J Pharmacol. 2017 Jun 5;804:111-116.
BACKGROUND: Endothelin receptor antagonists are approved for pulmonary arterial hypertension. Development of selective ETA-receptor antagonists over mixed or dual receptor antagonists has depended on a range of receptor binding assays, second messenger assays and functional blood vessel assays. This study compared the 3 clinically-approved endothelin receptor antagonists in assays of human isolated pulmonary and radial arteries in vitro. METHODS: Human isolated pulmonary (i.d. 5.5mm) and human radial (i.d. 3.23mm) artery ring segments were mounted in organ baths for isometric force measurement. Single concentration-contraction curves to endothelin-1 were constructed in the absence or presence of bosentan (1-10microM), Macitentan (0.03-0.3microM) or ambrisentan (0.1-1microM). RESULTS: All 3 endothelin antagonists caused competitive rightward shifts in the endothelin-1 concentration-response curves in both arteries. The Clark plot and analysis gave the following pKB values: bosentan, pulmonary artery 6.28+/-0.13 and radial artery 6.04+/-0.10; Macitentan, pulmonary artery 8.02+/-0.13 and radial artery 7.49+/-0.08; and ambrisentan, pulmonary artery 7.38+/-0.13 and radial artery 6.96+/-0.10. CONCLUSIONS: Noting the maximum plasma levels attained from recommended oral doses of each antagonist in volunteers, the pKB findings here show that there would be significant antagonism of endothelin-1 contraction in the pulmonary and radial arteries at therapeutic plasma levels. This functional assay confirms in human tissue that much higher plasma concentrations of endothelin-1 receptor antagonists are required to be effective than those predicted from binding or other biochemical assays.


