WAY 200070CAS# 440122-66-7 |
- Batimastat (BB-94)
Catalog No.:BCC1223
CAS No.:130370-60-4
- GM 6001
Catalog No.:BCC2119
CAS No.:142880-36-2
- CTS-1027
Catalog No.:BCC1502
CAS No.:193022-04-7
- PD 166793
Catalog No.:BCC2376
CAS No.:199850-67-4
- CP 471474
Catalog No.:BCC2373
CAS No.:210755-45-6
- CL 82198 hydrochloride
Catalog No.:BCC2372
CAS No.:307002-71-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 440122-66-7 | SDF | Download SDF |
| PubChem ID | 9861330 | Appearance | Powder |
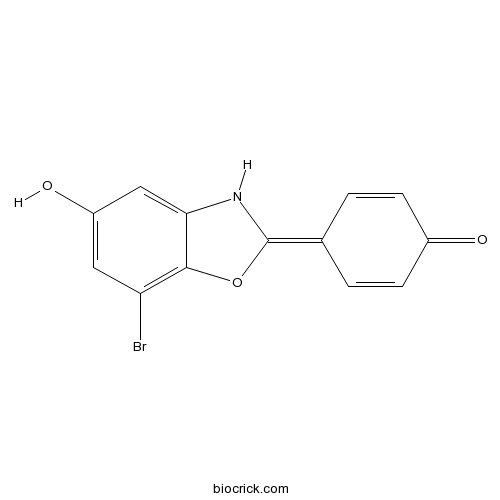
| Formula | C13H8BrNO3 | M.Wt | 306.11 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 31 mg/mL (101.27 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | 4-(7-bromo-5-hydroxy-3H-1,3-benzoxazol-2-ylidene)cyclohexa-2,5-dien-1-one | ||
| SMILES | C1=CC(=O)C=CC1=C2NC3=CC(=CC(=C3O2)Br)O | ||
| Standard InChIKey | IVFWABSHZFOMIG-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C13H8BrNO3/c14-10-5-9(17)6-11-12(10)18-13(15-11)7-1-3-8(16)4-2-7/h1-6,15,17H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective ERβ receptor agonist that displays 68-fold selectivity over ERα (EC50 values are 2 and 155 nM for ERβ and ERα respectively). Displays antianxiolytic and antidepressive effects in vivo. |

WAY 200070 Dilution Calculator

WAY 200070 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.2668 mL | 16.334 mL | 32.668 mL | 65.336 mL | 81.67 mL |
| 5 mM | 0.6534 mL | 3.2668 mL | 6.5336 mL | 13.0672 mL | 16.334 mL |
| 10 mM | 0.3267 mL | 1.6334 mL | 3.2668 mL | 6.5336 mL | 8.167 mL |
| 50 mM | 0.0653 mL | 0.3267 mL | 0.6534 mL | 1.3067 mL | 1.6334 mL |
| 100 mM | 0.0327 mL | 0.1633 mL | 0.3267 mL | 0.6534 mL | 0.8167 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- K 579
Catalog No.:BCC2364
CAS No.:440100-64-1
- Trifluoperazine 2HCl
Catalog No.:BCC4384
CAS No.:440-17-5
- Isoscabertopin
Catalog No.:BCN4634
CAS No.:439923-16-7
- Gnetifolin M
Catalog No.:BCN3394
CAS No.:439900-84-2
- NPY 5RA972
Catalog No.:BCC7747
CAS No.:439861-56-0
- BMS-509744
Catalog No.:BCC1424
CAS No.:439575-02-7
- ITK inhibitor
Catalog No.:BCC1662
CAS No.:439574-61-5
- GW 627368
Catalog No.:BCC7961
CAS No.:439288-66-1
- Lasmiditan
Catalog No.:BCC4077
CAS No.:439239-90-4
- Bay 60-7550
Catalog No.:BCC1405
CAS No.:439083-90-6
- Afatinib
Catalog No.:BCC3656
CAS No.:439081-18-2
- O-2093
Catalog No.:BCC7070
CAS No.:439080-01-0
- 3-Phenyl-2-propen-1-ol
Catalog No.:BCN5493
CAS No.:4407-36-7
- Benzoin oxime
Catalog No.:BCC8858
CAS No.:441-38-3
- Eribulin mesylate
Catalog No.:BCC5173
CAS No.:441045-17-6
- 2-(Cyanomethyl)benzimidazole
Catalog No.:BCC8483
CAS No.:4414-88-4
- 6-Acetylacteoside
Catalog No.:BCC8109
CAS No.:441769-43-3
- Macitentan
Catalog No.:BCC1142
CAS No.:441798-33-0
- Harmine
Catalog No.:BCN5494
CAS No.:442-51-3
- Clemizole
Catalog No.:BCC1485
CAS No.:442-52-4
- Aspertine C
Catalog No.:BCN2153
CAS No.:442155-62-6
- CK-636
Catalog No.:BCC5107
CAS No.:442632-72-6
- CK 666
Catalog No.:BCC6088
CAS No.:442633-00-3
- JW 67
Catalog No.:BCC6251
CAS No.:442644-28-2
WAY-200070, a selective agonist of estrogen receptor beta as a potential novel anxiolytic/antidepressant agent.[Pubmed:18423777]
Neuropharmacology. 2008 Jun;54(7):1136-42.
Recent studies have reported that estrogen has antidepressant-like effects in animal models. In this study we used the highly selective ER beta agonist, WAY-200070, to examine the role of ER beta activation on brain neurochemistry and activity in antidepressant and anxiolytic models in male mice. Within 15 min of administration, WAY-200070 (30 mg/kg s.c.) caused the nuclear translocation of striatal ER beta receptors from the cytosol. WAY-200070 also increased c-fos activation 4h, but not 15 min after administration. Both nuclear translocation and c-fos induction effects of WAY-200070 demonstrate that WAY-200070 has bound to estrogen receptors and triggered downstream events. The absence of these effects in the ER beta KO mice confirms that WAY-200070 was targeting ER beta. Administration of WAY-200070 (30 mg/kg s.c.) produced a delayed approximately 50% increase in dopamine in the striatum of wild type mice. The effect was significant and maintained from 90 to 240 min. This increase was absent in ER beta KO mice. In wild type mice, WAY-200070 (30 mg/kg s.c.) also produced a delayed and transient approximately 100% increase in 5-HT. To further investigate the role of ER beta receptors on serotonergic function, 5-HTP accumulation was measured. ER beta KO mice were found to have reduced frontal cortex levels of 5-HTP, indicating reduced tryptophan hydroxylase activity. WAY-200070 (3-30 mg/kg s.c.) was also tested in behavioural models. WAY-200070 (30 mg/kg s.c.) reduced immobility time in the mouse tail suspension test indicating an antidepressant-like effect. WAY-200070 (30 mg/kg) showed anxiolytic-like effects in the four-plate test (increased punished crossings) and stress-induced hyperthermia (attenuation of hyperthermic response). The effects of the selective ER beta agonist, WAY-200070, on dopamine and serotonin, the anxiolytic-like and antidepressant-like effects as well as the genotype specific effects on neurochemistry support that positive modulation of ER beta function may provide a novel treatment for affective disorders.
Effects of lorazepam and WAY-200070 in larval zebrafish light/dark choice test.[Pubmed:25842247]
Neuropharmacology. 2015 Aug;95:226-33.
Zebrafish larvae spend more time in brightly illuminated area when placed in a light/dark testing environment. Here we report that the anxiolytic drugs lorazepam and diazepam increased the time larval fish spent in the dark compartment in the light/dark test. Lorazepam did not affect the visual induced optokinetic response of larval fish. Gene expression levels of c-fos and crh were significantly increased in the hypothalamus of fish larvae underwent light/dark choice behavior, whilst lorazepam treatment alleviated the increased c-fos and crh expressions. Furthermore, we found estrogen receptor beta gene expression level was increased in fish larvae underwent light/dark choice. We next examined effects of estrogen receptor modulators (estradiol, BPA, PHTPP, and WAY-200070) in the light/dark test. We identified WAY-200070, a highly selective ERbeta agonist significantly altered the light/dark choice behavior of zebrafish larvae. Further investigation showed WAY-200070 treatment caused a reduction of crh expression level in the hypothalamus, suggesting activation of ERbeta signaling attenuate the stress response. Interestingly, WAY-200070 treatment caused marked increase of c-fos expression in the habenula of fish larvae underwent behavior test. These results suggest WAY-200070 activation of ERbeta mediated signaling may regulate anxiety related behavior in zebrafish through modulation of neuronal activity in habenula.
Differential effects of estrogen receptor alpha and beta specific agonists on social learning of food preferences in female mice.[Pubmed:18004284]
Neuropsychopharmacology. 2008 Sep;33(10):2362-75.
There is an evolutionary advantage to learning food preferences from conspecifics, as social learning allows an individual to bypass the risks associated with trial and error individual learning. The social transmission of food preferences (STFP) paradigm examines this advantage. Females in the proestrus and diestrus phases of the estrous cycle show a prolonged preference for the demonstrated food relative to estrus and ovariectomized females. Additionally, both estrogen receptor alpha (ERalpha) and estrogen receptor beta (ERbeta) knockout mice show impaired social recognition, which suggests that both receptors may be involved in other types of socially dependent learning, including the STFP. The present study investigated the effect of the ERalpha selective agonist PPT (1,3,5-tris(4-hydroxyphenyl)-4-propyl-1H-pyrazole) and the ERbeta selective agonist WAY-200070 (7-Bromo-2-(4-hydroxyphenyl)-1,3-benzoxazol-5-ol) on the STFP. Results showed that ovariectomized (ovx) mice treated with PPT failed to learn the socially acquired preference, while WAY-200070-treated ovx mice showed a two-fold prolonged preference for the food eaten by their demonstrator. The effects of PPT on the socially acquired food preference cannot be explained by effects on the total food intake of the groups or on the type of interaction with the demonstrator mouse. The effects of WAY-200070 may be partially due to effects on Submissive Behavior. The higher WAY-200070 doses produced prolonged preferences similar to those seen previously in intact female mice during the proestrus and diestrus phases. This suggests that the estrous cycle's effects on social learning may be due to the action of ERbeta on Submissive Behavior, or to ERbeta countering that of ERalpha.
Design and synthesis of aryl diphenolic azoles as potent and selective estrogen receptor-beta ligands.[Pubmed:15456246]
J Med Chem. 2004 Oct 7;47(21):5021-40.
New diphenolic azoles as highly selective estrogen receptor-beta agonists are reported. The more potent and selective analogues of these series have comparable binding affinities for ERbeta as the natural ligand 17beta-estradiol but are >100-fold selective over ERalpha. Our design strategy not only followed a traditional SAR approach but also was supported by X-ray structures of ERbeta cocrystallized with various ligands as well as molecular modeling studies. These strategies enabled us to take advantage of a single conservative residue substitution in the ligand-binding pocket, ERalpha Met(421) --> ERbeta Ile(373), to optimize ERbeta selectivity. The 7-position-substituted benzoxazoles (Table 5) were the most selective ligands of both azole series, with ERB-041 (117) being >200-fold selective for ERbeta. The majority of ERbeta selective agonists tested that were at least approximately 50-fold selective displayed a consistent in vivo profile: they were inactive in several models of classic estrogen action (uterotrophic, osteopenia, and vasomotor instability models) and yet were active in the HLA-B27 transgenic rat model of inflammatory bowel disease. These data suggest that ERbeta-selective agonists are devoid of classic estrogenic effects and may offer a novel therapy to treat certain inflammatory conditions.


