Kanzonol CCAS# 151135-82-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 151135-82-9 | SDF | Download SDF |
| PubChem ID | 5316802 | Appearance | Powder |
| Formula | C25H28O4 | M.Wt | 392.49 |
| Type of Compound | Chalcones | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
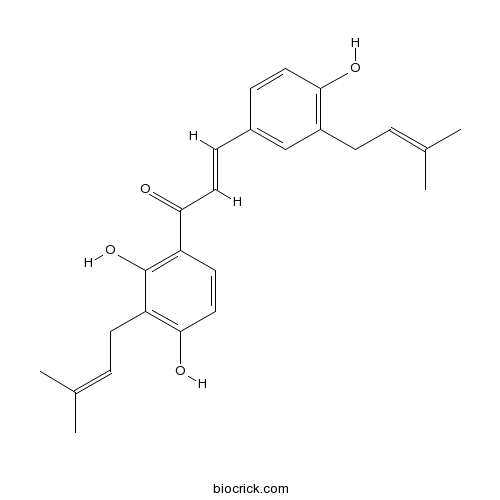
| Chemical Name | (E)-1-[2,4-dihydroxy-3-(3-methylbut-2-enyl)phenyl]-3-[4-hydroxy-3-(3-methylbut-2-enyl)phenyl]prop-2-en-1-one | ||
| SMILES | CC(=CCC1=C(C=CC(=C1)C=CC(=O)C2=C(C(=C(C=C2)O)CC=C(C)C)O)O)C | ||
| Standard InChIKey | CBGDCCSHOGQUSW-MDWZMJQESA-N | ||
| Standard InChI | InChI=1S/C25H28O4/c1-16(2)5-9-19-15-18(7-12-22(19)26)8-13-23(27)21-11-14-24(28)20(25(21)29)10-6-17(3)4/h5-8,11-15,26,28-29H,9-10H2,1-4H3/b13-8+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Kanzonol C shows potent PTP1B inhibitory activity (IC50 value of 0.31-0.97uM). 2. Kanzonol C has antimicrobial activity, it prevented the growth of all the 22 tested microbial species. 3. Kanzonol C has chemopreventive activity,it shows the inhibition of matrix metalloproteinase (MMP)-2 secretion from brain tumor-derived glioblastoma cells. |
| Targets | NO | NF-kB | Tyrosinase | Antifection |

Kanzonol C Dilution Calculator

Kanzonol C Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.5478 mL | 12.7392 mL | 25.4784 mL | 50.9567 mL | 63.6959 mL |
| 5 mM | 0.5096 mL | 2.5478 mL | 5.0957 mL | 10.1913 mL | 12.7392 mL |
| 10 mM | 0.2548 mL | 1.2739 mL | 2.5478 mL | 5.0957 mL | 6.3696 mL |
| 50 mM | 0.051 mL | 0.2548 mL | 0.5096 mL | 1.0191 mL | 1.2739 mL |
| 100 mM | 0.0255 mL | 0.1274 mL | 0.2548 mL | 0.5096 mL | 0.637 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Ginsenoside Rh7
Catalog No.:BCN8656
CAS No.:343780-68-7
- 5-Ethoxy-10-Gingerol
Catalog No.:BCN8655
CAS No.:121771-98-0
- Angelicolide
Catalog No.:BCN8654
CAS No.:90826-58-7
- 11alpha-Methoxysaikosaponin F
Catalog No.:BCN8653
CAS No.:104109-37-7
- Daidzein diacetate
Catalog No.:BCN8652
CAS No.:3682-01-7
- 1-Methyl-6-oxopyridine-3-carboxylic acid
Catalog No.:BCN8651
CAS No.:3719-45-7
- Nortracheloside
Catalog No.:BCN8650
CAS No.:33464-78-7
- Euphorbia factor L7b
Catalog No.:BCN8649
CAS No.:93550-95-9
- Bryodulcosigenin
Catalog No.:BCN8648
CAS No.:4965-97-3
- Nephthenol
Catalog No.:BCN8647
CAS No.:53915-41-6
- 14,15beta-Dihydroxyklaineanone
Catalog No.:BCN8646
CAS No.:137359-82-1
- 1''-O-beta-D-glucopyranosylformoside
Catalog No.:BCN8645
CAS No.:148245-77-6
- Nuezhenidic acid
Catalog No.:BCN8658
CAS No.:183238-67-7
- Vinaginsenoside R4
Catalog No.:BCN8659
CAS No.:156009-80-2
- Bacopaside I
Catalog No.:BCN8660
CAS No.:382148-47-2
- Glicoricone
Catalog No.:BCN8661
CAS No.:161099-37-2
- Angelicide
Catalog No.:BCN8662
CAS No.:92935-94-9
- Aromadendrene
Catalog No.:BCN8663
CAS No.:489-39-4
- Parvisoflavanone
Catalog No.:BCN8664
CAS No.:49776-79-6
- Lappaol F
Catalog No.:BCN8665
CAS No.:69394-17-8
- 4''-methyloxy-Daidzin
Catalog No.:BCN8667
CAS No.:1195968-02-5
- 4''-methyloxy-Genistin
Catalog No.:BCN8668
CAS No.:950910-16-4
- Ophiopogonanone B
Catalog No.:BCN8669
CAS No.:1316759-83-7
- Moscatin
Catalog No.:BCN8670
CAS No.:108335-06-4
Screening for bioactive natural products from a 67-compound library of Glycyrrhiza inflata.[Pubmed:28522265]
Bioorg Med Chem. 2017 Jul 15;25(14):3706-3713.
Licorice shows a variety of pharmacological activities. This work aims to discover bioactive natural products from one botanical source of licorice, Glycyrrhiza inflata. A total of 67 free phenolics were isolated to form a compound library. Based on the bioactivities of licorice, these compounds were screened using cell- or enzyme-based bioassay methods. A total of 11 compounds exhibited potent cytotoxic activities against three human cancer cell lines (HepG2, SW480 and MCF7), while showed little toxicity on human normal cell lines LO2 and HEK293T. A number of chalcones showed remarkable anti-inflammatory activities. Among them, 2 (licochalcone B, IC50 8.78muM), 10 (licoagrochalcone C, IC50 9.35muM) and 13 (licochalcone E, IC50 9.09muM) exhibited the most potent inhibitory activities on LPS-induced NO production, whereas 1, 8, 10, 12 and 13 (IC50 13.9, 7.27, 2.44, 6.67 and 3.83muM) showed potent inhibitory activities on NF-kappaB transcription. Nine prenylated phenolics were found to be PTP1B inhibitors. Particularly, licoagrochalcone A (4), Kanzonol C (7), 2'-hydroxyisolupalbigenin (35), gancaonin Q (45), glisoflavanone (50) and glabrol (53) showed IC50 values of 0.31-0.97muM. Compounds 24 (semilicoisoflavone B, IC50 0.25muM), 26 (allolicoisoflavone B, IC50 0.80muM) and 64 (glabridin, IC50 0.10muM) showed noticeable tyrosinase inhibitory activities. Most of the above bioactive compounds were reported for the first time.
Antimicrobial activity of the crude extracts and five flavonoids from the twigs of Dorstenia barteri (Moraceae).[Pubmed:18280679]
J Ethnopharmacol. 2008 Mar 28;116(3):483-9.
The aim of this study was to evaluate the antimicrobial activity of the crude extract of the twigs of Dorstenia barteri (DBT) as well as that of four of the five flavonoids isolated from this extract. Gram-positive bacteria (six species), Gram-negative bacteria (12 species) and fungi (four species) were used. The agar disc diffusion test was used to determine the sensitivity of the tested samples while the well micro-dilution was used to determine the minimal inhibition concentrations (MIC) and the minimal microbicidal concentration (MMC) of the active samples. The results of the disc diffusion assay showed that DBT, isobavachalcone (1), and Kanzonol C (4) prevented the growth of all the 22 tested microbial species. Other compounds showed selective activity. The inhibitory activity of the most active compounds namely compounds 1 and 4 was noted on 86.4% of the tested microorganisms and that of 4-hydroxylonchocarpin (3) was observed on 72.7%. This lowest MIC value of 19.06microg/ml was observed with the crude extract on seven microorganisms namely Citrobacter freundii, Enterobacter aerogens, Proteus mirabilis, Proteus vulgaris, Bacillus megaterium, Bacillus stearothermophilus and Candida albicans. For the tested compounds, the lowest MIC value of 0.3microg/ml (on six of the 22 organisms tested) was obtained only with compound 1, which appeared as the most active compound. This lowest MIC value (0.3microg/ml) is about 4-fold lower than that of the RA, indicating the powerful and very interesting antimicrobial potential of isobavachalcone (1). The antimicrobial activities of DBT, as well as that of compounds 1, 3, 4, amentoflavone (5) are being reported for the first time. The overall results provide promising baseline information for the potential use of the crude extracts from DBT as well as some of the isolated compounds in the treatment of bacterial and fungal infections.


