Vinaginsenoside R4CAS# 156009-80-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 156009-80-2 | SDF | Download SDF |
| PubChem ID | 15940177 | Appearance | Powder |
| Formula | C48H82O19 | M.Wt | 963.16 |
| Type of Compound | Triterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
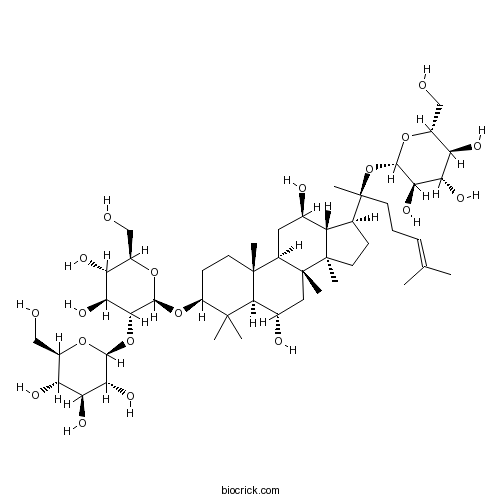
| Chemical Name | (2S,3R,4S,5S,6R)-2-[(2R,3R,4S,5S,6R)-2-[[(3S,5R,6S,8R,9R,10R,12R,13R,14R,17S)-6,12-dihydroxy-4,4,8,10,14-pentamethyl-17-[(2S)-6-methyl-2-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxyhept-5-en-2-yl]-2,3,5,6,7,9,11,12,13,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-3-yl]oxy]-4,5-dihydroxy-6-(hydroxymethyl)oxan-3-yl]oxy-6-(hydroxymethyl)oxane-3,4,5-triol | ||
| SMILES | CC(=CCCC(C)(C1CCC2(C1C(CC3C2(CC(C4C3(CCC(C4(C)C)OC5C(C(C(C(O5)CO)O)O)OC6C(C(C(C(O6)CO)O)O)O)C)O)C)O)C)OC7C(C(C(C(O7)CO)O)O)O)C | ||
| Standard InChIKey | UOJAEODBOCLNBU-GYMUUCMZSA-N | ||
| Standard InChI | InChI=1S/C48H82O19/c1-21(2)10-9-13-48(8,67-42-38(61)35(58)32(55)26(19-50)63-42)22-11-15-46(6)30(22)23(52)16-28-45(5)14-12-29(44(3,4)40(45)24(53)17-47(28,46)7)65-43-39(36(59)33(56)27(20-51)64-43)66-41-37(60)34(57)31(54)25(18-49)62-41/h10,22-43,49-61H,9,11-20H2,1-8H3/t22-,23+,24-,25+,26+,27+,28+,29-,30-,31+,32+,33+,34-,35-,36-,37+,38+,39+,40-,41-,42-,43-,45+,46+,47+,48-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Vinaginsenoside R4 has melanogenic inhibitory activity , it may have potential as a new skin whitening compound. |

Vinaginsenoside R4 Dilution Calculator

Vinaginsenoside R4 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.0382 mL | 5.1912 mL | 10.3825 mL | 20.765 mL | 25.9562 mL |
| 5 mM | 0.2076 mL | 1.0382 mL | 2.0765 mL | 4.153 mL | 5.1912 mL |
| 10 mM | 0.1038 mL | 0.5191 mL | 1.0382 mL | 2.0765 mL | 2.5956 mL |
| 50 mM | 0.0208 mL | 0.1038 mL | 0.2076 mL | 0.4153 mL | 0.5191 mL |
| 100 mM | 0.0104 mL | 0.0519 mL | 0.1038 mL | 0.2076 mL | 0.2596 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Nuezhenidic acid
Catalog No.:BCN8658
CAS No.:183238-67-7
- Kanzonol C
Catalog No.:BCN8657
CAS No.:151135-82-9
- Ginsenoside Rh7
Catalog No.:BCN8656
CAS No.:343780-68-7
- 5-Ethoxy-10-Gingerol
Catalog No.:BCN8655
CAS No.:121771-98-0
- Angelicolide
Catalog No.:BCN8654
CAS No.:90826-58-7
- 11alpha-Methoxysaikosaponin F
Catalog No.:BCN8653
CAS No.:104109-37-7
- Daidzein diacetate
Catalog No.:BCN8652
CAS No.:3682-01-7
- 1-Methyl-6-oxopyridine-3-carboxylic acid
Catalog No.:BCN8651
CAS No.:3719-45-7
- Nortracheloside
Catalog No.:BCN8650
CAS No.:33464-78-7
- Euphorbia factor L7b
Catalog No.:BCN8649
CAS No.:93550-95-9
- Bryodulcosigenin
Catalog No.:BCN8648
CAS No.:4965-97-3
- Nephthenol
Catalog No.:BCN8647
CAS No.:53915-41-6
- Bacopaside I
Catalog No.:BCN8660
CAS No.:382148-47-2
- Glicoricone
Catalog No.:BCN8661
CAS No.:161099-37-2
- Angelicide
Catalog No.:BCN8662
CAS No.:92935-94-9
- Aromadendrene
Catalog No.:BCN8663
CAS No.:489-39-4
- Parvisoflavanone
Catalog No.:BCN8664
CAS No.:49776-79-6
- Lappaol F
Catalog No.:BCN8665
CAS No.:69394-17-8
- 4''-methyloxy-Daidzin
Catalog No.:BCN8667
CAS No.:1195968-02-5
- 4''-methyloxy-Genistin
Catalog No.:BCN8668
CAS No.:950910-16-4
- Ophiopogonanone B
Catalog No.:BCN8669
CAS No.:1316759-83-7
- Moscatin
Catalog No.:BCN8670
CAS No.:108335-06-4
- Epimagnolin B
Catalog No.:BCN8671
CAS No.:1134188-26-3
- Resveratroloside
Catalog No.:BCN8672
CAS No.:38963-95-0
The potential of minor ginsenosides isolated from the leaves of Panax ginseng as inhibitors of melanogenesis.[Pubmed:25590297]
Int J Mol Sci. 2015 Jan 13;16(1):1677-90.
Three minor ginsenosides, namely, ginsenoside Rh6 (1), vina-ginsenoside R4 (2) and vina-ginsenoside R13 (3), were isolated from the leaves of hydroponic Panax ginseng. The chemical structures were determined based on spectroscopic methods, including fast atom bombardment mass spectroscopy (FAB-MS), 1D-nuclear magnetic resonance (NMR), 2D-NMR, and, infrared (IR) spectroscopy. The melanogenic inhibitory activity of compounds 1, 2 and 3 was 23.9%, 27.8% and 35.2%, respectively, at a concentration of 80 microM. Likewise, the three compounds showed inhibitory activity on body pigmentation on a zebrafish model, which is commonly used as a model for biomedical or cosmetic research. These results from in vitro and in vivo systems suggest that the three aforementioned compounds isolated from Panax ginseng may have potential as new skin whitening compounds.
Saponins from Vietnamese Ginseng, Panax vietnamensis HA et Grushv. Collected in central Vietnam. II.[Pubmed:8124758]
Chem Pharm Bull (Tokyo). 1994 Jan;42(1):115-22.
Further investigation on the saponin composition of rhizomes and roots of Panax vietnamensis HA et GRUSHV. has resulted in the isolation and structural elucidation of seven new dammarane saponins named vina-ginsenosides-R3 (12), -R4 (11), -R5 (16), -R6 (17), -R7 (6), -R8 (20), -R9 (22), together with the identification of six known saponins including 20-gluco-ginsenoside-Rf (10), ginsenoside-Rc (4), notoginsenoside-R6 (9), quinquenoside-R1 (5), gypenoside XVII (2) and majoroside F1 (21). The structures of the novel saponins were established on the basis of chemical and spectral evidence. Vina-ginsenoside-R3 is the first naturally occurring glycoside of dammarenediol II, while vina-ginsenosides-R5 and -R6, two ocotillol-type saponins, are two other examples of saponins having the rare alpha-glucosyl linkage.


