ResveratrolosideCAS# 38963-95-0 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 38963-95-0 | SDF | Download SDF |
| PubChem ID | 5322089 | Appearance | Powder |
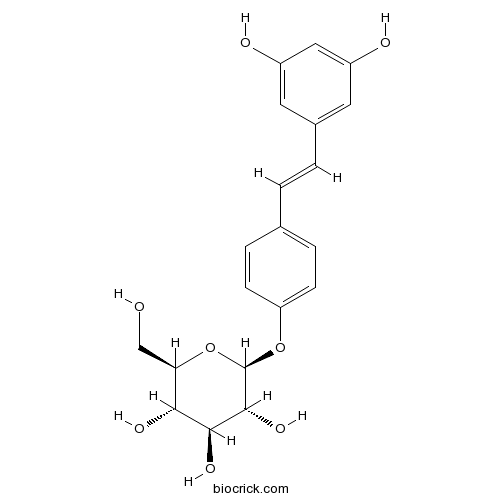
| Formula | C20H22O8 | M.Wt | 390.38 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (2S,3R,4S,5S,6R)-2-[4-[(E)-2-(3,5-dihydroxyphenyl)ethenyl]phenoxy]-6-(hydroxymethyl)oxane-3,4,5-triol | ||
| SMILES | C1=CC(=CC=C1C=CC2=CC(=CC(=C2)O)O)OC3C(C(C(C(O3)CO)O)O)O | ||
| Standard InChIKey | RUOKEYJFAJITAG-CUYWLFDKSA-N | ||
| Standard InChI | InChI=1S/C20H22O8/c21-10-16-17(24)18(25)19(26)20(28-16)27-15-5-3-11(4-6-15)1-2-12-7-13(22)9-14(23)8-12/h1-9,16-26H,10H2/b2-1+/t16-,17-,18+,19-,20-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Resveratroloside has anti-inflammatory activity, it shows similar nitric oxide (NO) inhibition potency as that of piceid (the major constituent of P. cuspidatum). 2. Resveratroloside has cardioprotective effect. 3. Resveratroloside can inhibit hydroxylation of testosterone by CYP3A4. 4. Resveratroloside exhibits α-glucosidase inhibitory activity. |
| Targets | NO | P450 (e.g. CYP17) |

Resveratroloside Dilution Calculator

Resveratroloside Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.5616 mL | 12.808 mL | 25.6161 mL | 51.2321 mL | 64.0402 mL |
| 5 mM | 0.5123 mL | 2.5616 mL | 5.1232 mL | 10.2464 mL | 12.808 mL |
| 10 mM | 0.2562 mL | 1.2808 mL | 2.5616 mL | 5.1232 mL | 6.404 mL |
| 50 mM | 0.0512 mL | 0.2562 mL | 0.5123 mL | 1.0246 mL | 1.2808 mL |
| 100 mM | 0.0256 mL | 0.1281 mL | 0.2562 mL | 0.5123 mL | 0.6404 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Epimagnolin B
Catalog No.:BCN8671
CAS No.:1134188-26-3
- Moscatin
Catalog No.:BCN8670
CAS No.:108335-06-4
- Ophiopogonanone B
Catalog No.:BCN8669
CAS No.:1316759-83-7
- 4''-methyloxy-Genistin
Catalog No.:BCN8668
CAS No.:950910-16-4
- 4''-methyloxy-Daidzin
Catalog No.:BCN8667
CAS No.:1195968-02-5
- Lappaol F
Catalog No.:BCN8665
CAS No.:69394-17-8
- Parvisoflavanone
Catalog No.:BCN8664
CAS No.:49776-79-6
- Aromadendrene
Catalog No.:BCN8663
CAS No.:489-39-4
- Angelicide
Catalog No.:BCN8662
CAS No.:92935-94-9
- Glicoricone
Catalog No.:BCN8661
CAS No.:161099-37-2
- Bacopaside I
Catalog No.:BCN8660
CAS No.:382148-47-2
- Vinaginsenoside R4
Catalog No.:BCN8659
CAS No.:156009-80-2
- Pomiferin
Catalog No.:BCN8673
CAS No.:572-03-2
- Aurantio-obtusin beta-D-glucoside
Catalog No.:BCN8674
CAS No.:129025-96-3
- 13-Methylberberine
Catalog No.:BCN8675
CAS No.:54260-72-9
- Lappaol C
Catalog No.:BCN8676
CAS No.:64855-00-1
- Magnaldehyde B
Catalog No.:BCN8677
CAS No.:92829-72-6
- Flemiphilippinin A
Catalog No.:BCN8678
CAS No.:140366-64-9
- Kudinoside D
Catalog No.:BCN8679
CAS No.:173792-61-5
- Perisesaccharide C
Catalog No.:BCN8680
CAS No.:1311473-28-5
- Cafestol
Catalog No.:BCN8681
CAS No.:469-83-0
- Huzhangoside B
Catalog No.:BCN8682
CAS No.:94795-70-7
- Protohypericin
Catalog No.:BCN8683
CAS No.:548-03-8
- Glabrol
Catalog No.:BCN8684
CAS No.:59870-65-4
Analysis and functionality of major polyphenolic components of Polygonum cuspidatum (itadori).[Pubmed:23132312]
J Nutr Sci Vitaminol (Tokyo). 2012;58(4):278-86.
Polygonum cuspidatum has been broadly utilized as a herbal medicine in Asia, but the outline of the polyphenol compounds in the plant has not been characterized well. In the present study, the major polyphenolic components were isolated from the roots of P. cuspidatum, and identified as resveratrol and its glucosides, Resveratroloside and polydatin. On the other hand, chlorogenic acid was found to be one of the polyphenolic components in the leaves and stems of the plant. Next, we elucidated that resveratrol derivatives and chlorogenic acid exhibit alpha-glucosidase inhibitory activities, and Resveratroloside exhibits the same inhibitory activity as quercetin does. Furthermore, DPPH radical scavenging activities of antioxidants including resveratrol derivatives and chlorogenic acid derivatives were examined by initial rate analyses of their reactions. Subsequently, it was revealed that resveratrol derivatives have slow-acting effects on the radical scavenging activity and that chlorogenic acid derivatives exhibit very fast-acting effects.
Cardioprotective effect of resveratrol and resveratroloside.[Pubmed:23547903]
Cardiovasc Hematol Agents Med Chem. 2013 Sep;11(3):207-10.
Cardioprotective effect of resveratrol and Resveratroloside was determined in ischemia-reperfusion experiments on rats. It was found that single intraperitoneal administration of any compound (10 mg/kg) followed by 30-min ischemia and 120-min reperfusion resulted in statistically significant decrease of myocardial infarct area (55.0+/-4.0% for control group; 40.7+/-4.4% for the group 1 received resveratrol; 41.6+/-4.8% for the group 2 received Resveratroloside). The cardioprotective effect of Resveratroloside was detected for the first time.
Anti-inflammatory Activity of the Invasive Neophyte Polygonum Cuspidatum Sieb. and Zucc. (Polygonaceae) and the Chemical Comparison of the Invasive and Native Varieties with regard to Resveratrol.[Pubmed:24716176]
J Tradit Complement Med. 2013 Jul;3(3):182-7.
Polygonum cuspidatum Sieb. and Zucc. has been traditionally used as a member of many anti-inflammatory polyherbal formulations, but is now a widespread invasive neophyte in Europe and America. To discuss if the invasive variety is chemically identical to the native one in traditional medicine, the different constituents of the invasive variety compared to the native variety were isolated and their anti-inflammatory activity was tested. Resveratroloside and catechin-(4alpha-->8)-catechin, the newly found constituents in the invasive variety, have similar nitric oxide (NO) inhibition potency as that of piceid (the major constituent of P. cuspidatum), but the newly found major constituent, i.e., piceatannol glucoside, showed no apparent effect. On the other hand, as a marker, the total content of resveratrol in the methanol root extract after glucosidase hydrolysis was measured and compared between the invasive and native varieties. The total content of resveratrol measured in the root extracts of the Swiss sample was about 2.5 times less than that of the Chinese one. This study brings attention to the point that when the invasive variety of P. cuspidatum is used in traditional medicine, the chemical difference should be kept in mind.
Influence of lipophilicity on the interactions of hydroxy stilbenes with cytochrome P450 3A4.[Pubmed:15369802]
Biochem Biophys Res Commun. 2004 Oct 15;323(2):668-73.
Resveratrol, a polyphenol found in red wine, was recently suggested to act as an irreversible, mechanism-based inactivator of cytochrome P450 3A4 (CYP3A4). We found a significant inhibition of human CYP3A4-dependent transformation of cyclosporine by resveratrol, with IC50 = 4.5 microM. We studied the kinetics parameters of CYP3A4 transformation of resveratrol and structurally related, naturally occurring stilbenes. Resveratrol, piceid, Resveratroloside, 5,4'-dihydroxy-3-O-methoxystilbene, and 5,3-dihydroxy-4'-O-methoxystilbene were all shown to inhibit hydroxylation of testosterone by CYP3A4. Both methoxy-stilbenes had lower IC50 values, ranging from 0.43 to 0.47 microM, suggesting that lipophilicity rather than number or positions of free hydroxyls (3,5 or 5,4') determines the CYP3A4 inhibition capacity of polyphenols. In line with these findings, both glucosyl-stilbenes were found to be weak inhibitors of CYP3A4. The affinity of the enzyme towards methoxy-stilbenes, expressed as apparent Km, was indeed higher than those for the parent resveratrol and its glucosides, in CYP3A4 reaction mixtures. Vmax values were similar, except for piceid. These results support the role of lipophilicity in the interaction of polyphenols with CYP3A4. It is suggested that selective structural modifications of substrates add significantly to knowledge acquired through molecular modifications of the enzyme.


