PomiferinCAS# 572-03-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 572-03-2 | SDF | Download SDF |
| PubChem ID | 4871 | Appearance | Powder |
| Formula | C25H24O6 | M.Wt | 420.46 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
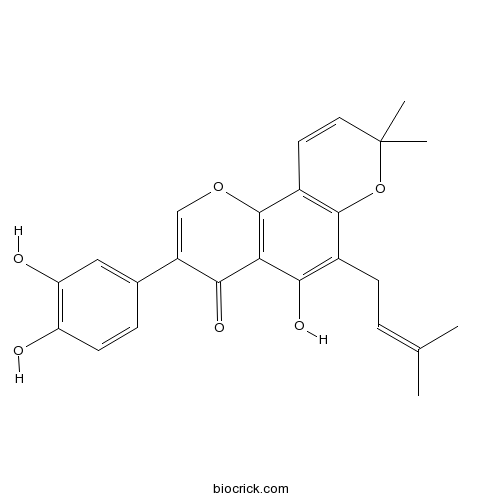
| Chemical Name | 3-(3,4-dihydroxyphenyl)-5-hydroxy-8,8-dimethyl-6-(3-methylbut-2-enyl)pyrano[2,3-h]chromen-4-one | ||
| SMILES | CC(=CCC1=C(C2=C(C3=C1OC(C=C3)(C)C)OC=C(C2=O)C4=CC(=C(C=C4)O)O)O)C | ||
| Standard InChIKey | GHCZYXUOYFOXIP-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C25H24O6/c1-13(2)5-7-15-21(28)20-22(29)17(14-6-8-18(26)19(27)11-14)12-30-24(20)16-9-10-25(3,4)31-23(15)16/h5-6,8-12,26-28H,7H2,1-4H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Pomiferin is a natural product inhibitor of carbonic anhydrase I and II isoenzymes. 2. Pomiferin has anticancer, antibacterial and antidiabetic properties. 3. Pomiferin has antiproliferative activity in normal (MCF-10A) and transformed (MCF-7) breast epithelial cells. 4. Pomiferin has anti-inflammatory and neuroprotective activities, it is effective in enhancing the activity of NSAID activated gene (NAG-1) at 2.5 pg/mL (1.5-1.8 fold increase) and inhibiting iNOS and NF-κB activity with IC50 values in the range of 6-13 ug/mL. 5. Pomiferin has protection on kidney ischaemia-reperfusion injury in rats. |
| Targets | PDE | NOS | NF-kB |

Pomiferin Dilution Calculator

Pomiferin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3783 mL | 11.8917 mL | 23.7835 mL | 47.567 mL | 59.4587 mL |
| 5 mM | 0.4757 mL | 2.3783 mL | 4.7567 mL | 9.5134 mL | 11.8917 mL |
| 10 mM | 0.2378 mL | 1.1892 mL | 2.3783 mL | 4.7567 mL | 5.9459 mL |
| 50 mM | 0.0476 mL | 0.2378 mL | 0.4757 mL | 0.9513 mL | 1.1892 mL |
| 100 mM | 0.0238 mL | 0.1189 mL | 0.2378 mL | 0.4757 mL | 0.5946 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Resveratroloside
Catalog No.:BCN8672
CAS No.:38963-95-0
- Epimagnolin B
Catalog No.:BCN8671
CAS No.:1134188-26-3
- Moscatin
Catalog No.:BCN8670
CAS No.:108335-06-4
- Ophiopogonanone B
Catalog No.:BCN8669
CAS No.:1316759-83-7
- 4''-methyloxy-Genistin
Catalog No.:BCN8668
CAS No.:950910-16-4
- 4''-methyloxy-Daidzin
Catalog No.:BCN8667
CAS No.:1195968-02-5
- Lappaol F
Catalog No.:BCN8665
CAS No.:69394-17-8
- Parvisoflavanone
Catalog No.:BCN8664
CAS No.:49776-79-6
- Aromadendrene
Catalog No.:BCN8663
CAS No.:489-39-4
- Angelicide
Catalog No.:BCN8662
CAS No.:92935-94-9
- Glicoricone
Catalog No.:BCN8661
CAS No.:161099-37-2
- Bacopaside I
Catalog No.:BCN8660
CAS No.:382148-47-2
- Aurantio-obtusin beta-D-glucoside
Catalog No.:BCN8674
CAS No.:129025-96-3
- 13-Methylberberine
Catalog No.:BCN8675
CAS No.:54260-72-9
- Lappaol C
Catalog No.:BCN8676
CAS No.:64855-00-1
- Magnaldehyde B
Catalog No.:BCN8677
CAS No.:92829-72-6
- Flemiphilippinin A
Catalog No.:BCN8678
CAS No.:140366-64-9
- Kudinoside D
Catalog No.:BCN8679
CAS No.:173792-61-5
- Perisesaccharide C
Catalog No.:BCN8680
CAS No.:1311473-28-5
- Cafestol
Catalog No.:BCN8681
CAS No.:469-83-0
- Huzhangoside B
Catalog No.:BCN8682
CAS No.:94795-70-7
- Protohypericin
Catalog No.:BCN8683
CAS No.:548-03-8
- Glabrol
Catalog No.:BCN8684
CAS No.:59870-65-4
- Isolindleyin
Catalog No.:BCN8685
CAS No.:87075-18-1
Potential of Horse Apple Isoflavones in Targeting Inflammation and Tau Protein Fibrillization.[Pubmed:26594763]
Nat Prod Commun. 2015 Sep;10(9):1577-80.
In our ongoing search for anti-inflammatory and neuroprotective agents of natural origin, the total methanolic extract (MPE) of horse apple (Maclura pomifera) and its two major prenylated isoflavones, osajin (OSA) and Pomiferin (POM), were evaluated in vitro for their ability to affect four mediators of inflammation and to inhibit tau protein fibrillization. The two isoflavones were effective in enhancing the activity of NSAID activated gene (NAG-1) at 2.5 pg/mL (1.5-1.8 fold increase) and inhibiting iNOS and NF-kappaB activity with IC50 values in the range of 6-13 microg/mL. Pomiferin also inhibited intracellular oxidative stress with IC50 of 3.3 microg/mL, while osajin did not show any effect. The extract activated NAG-1 and inhibited iNOS and oxidative stress without affecting NF-kappaB. As observed by transmission electron microscopy (TEM), MPE, OSA and POM also inhibited arachidonic acid-induced tau fibrillization in a concentration-dependent manner.
Effect of pomiferin administration on kidney ischaemia-reperfusion injury in rats.[Pubmed:21217877]
Interdiscip Toxicol. 2010 Jun;3(2):76-81.
The aim of the study was to analyse protective effects of different doses of Pomiferin in therapy of reperfusion injury. Rats were randomly divided into five groups (n=10). One group was intact. Three medicated groups and one placebo group were subjected to ischaemia and reperfusion of the left kidney. Pomiferin was administrated by single gastric gavage in 2 ml of 0.5% Avicel solution in doses of 5, 10 and 20 mg/kg. The placebo group was given only Avicel solution. On day 15, all the animals were exsanguinated and the reperfused kidneys were recovered. Selected biochemical markers were assessed in blood: antioxidative enzymes, total antioxidative capacity, malondialdehyde, creatinine, urea and uric acid. Creatinine, urea and total proteins were analysed in urine and 24-hour diuresis was recorded. The kidney tissue samples were used for histopathological examination.The results confirmed the expected protective effects of Pomiferin. Pomiferin supported defensive reactions of the body against free radicals (increased levels of superoxide dismutase, total antioxidative capacity), decreased lipid peroxidation (decreased malondialdehyde) and contributed to the recovery of kidney functions (creatinine and urea in blood). The best biochemical and histopathological results were achieved after Pomiferin administration in the dose of 5 mg/kg.
Antiproliferative activity of pomiferin in normal (MCF-10A) and transformed (MCF-7) breast epithelial cells.[Pubmed:22087557]
J Agric Food Chem. 2011 Dec 28;59(24):13328-36.
Pomiferin and osajin are prenylated isoflavones from Osage orange fruit that both have potent antioxidant activity in a variety of assays. Pomiferin, in particular, has strong activity against the superoxide anion in a photochemiluminescence (PCL) assay system. In vitro, Pomiferin, but not osajin, demonstrated selective antiproliferative activity against the tumorigenic breast epithelial cell line MCF-7 (IC(50) = 5.2 muM) with limited toxicity toward nontumorigenic breast epithelial cells (MCF-10A). The differential sensitivity of normal and tumorigenic cells to the antiproliferative action of Pomiferin was examined further by using cDNA microarrays. With a stringent cutoff of p < 0.01, a total of 94 genes were significantly differentially expressed between MCF-7 and MCF-10A cells; 80 up-regulated and 14 down-regulated when cells were exposed to 5 muM Pomiferin for 24 h. Fold changes by microarray analysis were confirmed using RT-qPCR, and the most significant changes were found with genes related to antioxidant enzymes. Genes involved in mitotic inhibition and apoptotic regulations were also found to be up-regulated. Pomiferin is therefore a good anticancer candidate agent that may be useful either alone or in combination with other therapeutic agents and, because of its selectivity toward tumor cells, likely to have fewer side effects that classic chemotherapy drugs.
Natural product inhibitors of carbonic anhydrase I and II isoenzymes: osajin and pomiferin.[Pubmed:28338341]
Arch Physiol Biochem. 2017 Oct;123(4):219-224.
The aim of this study is to purify carbonic anhydrase I and II isoenzymes from human erythrocyte, isolate two natural products osajin (OSJ) and Pomiferin (PMF) from Maclura pomifera fruits, and evaluate the in vitro effect of these natural metabolites on these isoenzymes. These natural products may be used as starting points for drug discovery (like drugs used in several therapeutic applications, including antiglaucoma activity). For the purification procedure, the Sepharose-4B-l-tyrosine-sulphonamide affinity chromatography was used. Column chromatography and thin layer chromatography methods were used for isolation of OSJ and PMF from M. pomifera fruits and their chemical structures were elucidated by IR, 1D, and 2D NMR methods. We compared inhibitory effects of these natural products with inhibitory effects of phenolic compounds and found that these products demonstrated average inhibition effects. We thought that this study will give inspiration to scientists interested in this issue.
Isoflavones from Maclura pomifera: structural elucidation and in silico evaluation of their interaction with PDE5.[Pubmed:28025893]
Nat Prod Res. 2017 Sep;31(17):1988-1994.
While osajin and Pomiferin are known for their anticancer, antibacterial and antidiabetic properties, scandenone and auriculasin have been proposed as anti-inflammatory and antinociceptive agents. Curiously, these two couples of molecules are, from a chemical point of view, structural isomers which can all be extracted from Maclura pomifera. Although previous works described, separately, the isolation in reasonable amounts of the sole osajin/Pomiferin couple or of scandenone/auriculasin, we report the extraction and characterization using direct spectral and chromatographical comparison of the four compounds. 2D NMR allowed to unambiguously assign the correct structures to the isomers. The compounds were screened in silico against PDE5 and their interaction pattern with the protein was compared with that of icarisid II, a natural PDE5 inhibitor.


