ML130 (Nodinitib-1)CAS# 799264-47-4 |
- TAK-438
Catalog No.:BCC1182
CAS No.:1260141-27-2
- Istaroxime
Catalog No.:BCC1660
CAS No.:203737-93-3
- Dynasore
Catalog No.:BCC1088
CAS No.:304448-55-3
- Istaroxime hydrochloride
Catalog No.:BCC1661
CAS No.:374559-48-5
- 20-HETE
Catalog No.:BCC1301
CAS No.:79551-86-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 799264-47-4 | SDF | Download SDF |
| PubChem ID | 1088438 | Appearance | Powder |
| Formula | C14H13N3O2S | M.Wt | 287.34 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | ML130; CID-1088438 | ||
| Solubility | DMSO : 20 mg/mL (69.60 mM; Need ultrasonic and warming) | ||
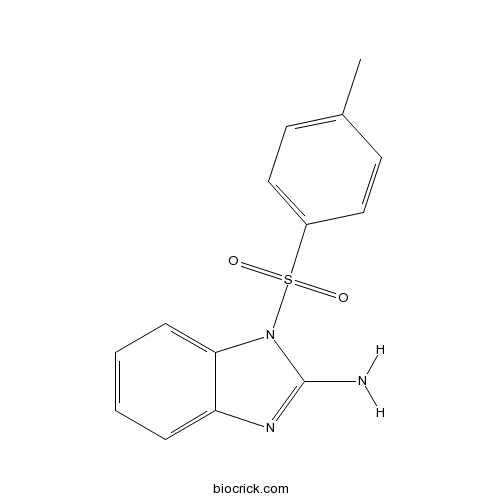
| Chemical Name | 1-(4-methylphenyl)sulfonylbenzimidazol-2-amine | ||
| SMILES | CC1=CC=C(C=C1)S(=O)(=O)N2C3=CC=CC=C3N=C2N | ||
| Standard InChIKey | SRFABRWQVPCPRG-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C14H13N3O2S/c1-10-6-8-11(9-7-10)20(18,19)17-13-5-3-2-4-12(13)16-14(17)15/h2-9H,1H3,(H2,15,16) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent and selective inhibitor of NOD1; displays 36-fold selectivity for NOD1 over NOD2 (IC50 values are 0.56 and >20 μM for NOD1 and NOD2 respectively). Inhibits NOD1-induced NF-κB activation in HEK293 cells with no cytotoxicity. Shown to alter subcellular targeting of NOD1; also thought to alter the conformation of NOD1 protein in vitro. |

ML130 (Nodinitib-1) Dilution Calculator

ML130 (Nodinitib-1) Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.4802 mL | 17.401 mL | 34.802 mL | 69.604 mL | 87.0049 mL |
| 5 mM | 0.696 mL | 3.4802 mL | 6.9604 mL | 13.9208 mL | 17.401 mL |
| 10 mM | 0.348 mL | 1.7401 mL | 3.4802 mL | 6.9604 mL | 8.7005 mL |
| 50 mM | 0.0696 mL | 0.348 mL | 0.696 mL | 1.3921 mL | 1.7401 mL |
| 100 mM | 0.0348 mL | 0.174 mL | 0.348 mL | 0.696 mL | 0.87 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
ML130 is a potent and selective inhibitor of NOD1 with IC50 of 0.56 μM, inhibits NF-κB activation, exhibits 36-fold selectivity over NOD2.
- Forsythoside A
Catalog No.:BCN1195
CAS No.:79916-77-1
- Simvastatin
Catalog No.:BCN2569
CAS No.:79902-63-9
- 1,2,3,6-Tetragalloylglucose
Catalog No.:BCN2159
CAS No.:79886-50-3
- Castanospermine
Catalog No.:BCC6783
CAS No.:79831-76-8
- Loratadine
Catalog No.:BCC1262
CAS No.:79794-75-5
- H-Hyp(tBu)-OH
Catalog No.:BCC3249
CAS No.:79775-07-8
- Levonorgestrel
Catalog No.:BCC4792
CAS No.:797-63-7
- Linifanib (ABT-869)
Catalog No.:BCC1261
CAS No.:796967-16-3
- Crassicauline A
Catalog No.:BCN2516
CAS No.:79592-91-9
- Alarelin Acetate
Catalog No.:BCC1336
CAS No.:79561-22-1
- Sertraline HCl
Catalog No.:BCC5059
CAS No.:79559-97-0
- 1,7-Diphenyl-4-hepten-3-one
Catalog No.:BCN3592
CAS No.:79559-59-4
- Idazoxan hydrochloride
Catalog No.:BCC6798
CAS No.:79944-56-2
- Boc-His(Bom)-OH
Catalog No.:BCC3400
CAS No.:79950-65-5
- Quinovic acid 3-O-beta-D-glucoside
Catalog No.:BCN4334
CAS No.:79955-41-2
- Fmoc-D-Ala-OH
Catalog No.:BCC3036
CAS No.:79990-15-1
- Blumeatin B
Catalog No.:BCN4335
CAS No.:79995-67-8
- Dapsone
Catalog No.:BCC5220
CAS No.:80-08-0
- Sulfamethoxypyridazine
Catalog No.:BCC4728
CAS No.:80-35-3
- Homatropine Methylbromide
Catalog No.:BCC4571
CAS No.:80-49-9
- Alpha-pinene
Catalog No.:BCN3855
CAS No.:80-56-8
- Tiglicacid
Catalog No.:BCN2976
CAS No.:80-59-1
- Sulfisoxazole Acetyl
Catalog No.:BCC5630
CAS No.:80-74-0
- Dihydrocholesterol
Catalog No.:BCN2749
CAS No.:80-97-7
Discovery and characterization of 2-aminobenzimidazole derivatives as selective NOD1 inhibitors.[Pubmed:21802003]
Chem Biol. 2011 Jul 29;18(7):825-32.
NLR family proteins play important roles in innate immune response. NOD1 (NLRC1) activates various signaling pathways including NF-kappaB in response to bacterial ligands. Hereditary polymorphisms in the NOD1 gene are associated with asthma, inflammatory bowel disease, and other disorders. Using a high throughput screening (HTS) assay measuring NOD1-induced NF-kappaB reporter gene activity, followed by multiple downstream counter screens that eliminated compounds impacting other NF-kappaB effectors, 2-aminobenzimidazole compounds were identified that selectively inhibit NOD1. Mechanistic studies of a prototypical compound, Nodinitib-1 (ML130; CID-1088438), suggest that these small molecules cause conformational changes of NOD1 in vitro and alter NOD1 subcellular targeting in cells. Altogether, this inaugural class of inhibitors provides chemical probes for interrogating mechanisms regulating NOD1 activity and tools for exploring the roles of NOD1 in various infectious and inflammatory diseases.
Identification of Inhibitors of NOD1-Induced Nuclear Factor-kappaB Activation.[Pubmed:22003428]
ACS Med Chem Lett. 2011 Oct 13;2(10):780-785.
NOD1 (nucleotide-binding oligomerization domain 1) protein is a member of the NLR (NACHT and leucine rich repeat domain containing proteins) protein family, which plays a key role in innate immunity as a sensor of specific microbial components derived from bacterial peptidoglycans and induction of inflammatory responses. Mutations in NOD proteins have been associated with various inflammatory diseases that affect NF-kappaB (nuclear factor kappaB) activity, a major signaling pathway involved in apoptosis, inflammation, and immune response. A luciferase-based reporter gene assay was utilized in a high-throughput screening program conducted under the NIH-sponsored Molecular Libraries Probe Production Center Network program to identify the active scaffolds. Herein, we report the chemical synthesis, structure-activity relationship studies, downstream counterscreens, secondary assay data, and pharmacological profiling of the 2-aminobenzimidazole lead (compound 1c, ML130) as a potent and selective inhibitor of NOD1-induced NF-kappaB activation.


