MuramineCAS# 2292-20-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 2292-20-8 | SDF | Download SDF |
| PubChem ID | 288122 | Appearance | Powder |
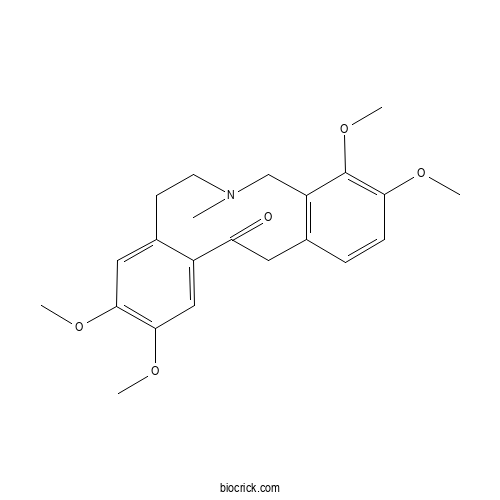
| Formula | C22H27NO5 | M.Wt | 385.5 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Synonyms | Cryptopalmatine | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 3,4,10,11-tetramethoxy-6-methyl-5,7,8,14-tetrahydrobenzo[e][2]benzazecin-13-one | ||
| SMILES | CN1CCC2=CC(=C(C=C2C(=O)CC3=C(C1)C(=C(C=C3)OC)OC)OC)OC | ||
| Standard InChIKey | HUIJAZQRYSCNED-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C22H27NO5/c1-23-9-8-15-11-20(26-3)21(27-4)12-16(15)18(24)10-14-6-7-19(25-2)22(28-5)17(14)13-23/h6-7,11-12H,8-10,13H2,1-5H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Muramine Dilution Calculator

Muramine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.594 mL | 12.9702 mL | 25.9403 mL | 51.8807 mL | 64.8508 mL |
| 5 mM | 0.5188 mL | 2.594 mL | 5.1881 mL | 10.3761 mL | 12.9702 mL |
| 10 mM | 0.2594 mL | 1.297 mL | 2.594 mL | 5.1881 mL | 6.4851 mL |
| 50 mM | 0.0519 mL | 0.2594 mL | 0.5188 mL | 1.0376 mL | 1.297 mL |
| 100 mM | 0.0259 mL | 0.1297 mL | 0.2594 mL | 0.5188 mL | 0.6485 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Uvamalol D
Catalog No.:BCN0495
CAS No.:545404-02-2
- 6-O-Veratroylcatalpol
Catalog No.:BCN0494
CAS No.:56973-43-4
- Piceatannol 4'-O-glucoside
Catalog No.:BCN0493
CAS No.:116181-54-5
- Piperchabamide B
Catalog No.:BCN0492
CAS No.:807618-21-9
- Tsaokoin
Catalog No.:BCN0491
CAS No.:343605-41-4
- Apigenin 7,4'-di-O-alloside
Catalog No.:BCN0490
CAS No.:95693-63-3
- 4''-O-Methylcatalposide
Catalog No.:BCN0489
CAS No.:887140-17-2
- Piperdardine
Catalog No.:BCN0488
CAS No.:188426-70-2
- 5-Hydroxy-6,7,3',4'-tetramethoxyflavone
Catalog No.:BCN0487
CAS No.:21763-80-4
- Verproside
Catalog No.:BCN0486
CAS No.:50932-20-2
- Marionol
Catalog No.:BCN0485
CAS No.:65602-55-3
- Acrovestone
Catalog No.:BCN0484
CAS No.:24177-16-0
- Sarmentine
Catalog No.:BCN0497
CAS No.:78910-33-5
- 5α,8α-Epidioxyergost-6-en-3β-ol
Catalog No.:BCN0498
CAS No.:82227-99-4
- Guineensine
Catalog No.:BCN0499
CAS No.:55038-30-7
- Retrofractamide B
Catalog No.:BCN0500
CAS No.:54794-74-0
- Chingchengenamide A
Catalog No.:BCN0501
CAS No.:139906-29-9
- Isoorientin 2''-O-rhamnoside
Catalog No.:BCN0502
CAS No.:50980-94-4
- 2E,4E-Decadienoylpiperidide
Catalog No.:BCN0503
CAS No.:42997-42-2
- 2E-Decenoylpiperidide
Catalog No.:BCN0504
CAS No.:147030-02-2
- Piperanine
Catalog No.:BCN0505
CAS No.:23512-46-1
- (3R,5R)-1-(4-Hydroxyphenyl)-7-phenylheptane-3,5-diol
Catalog No.:BCN0506
CAS No.:112494-44-7
- Alpinin A
Catalog No.:BCN0507
CAS No.:2151847-03-7
- 4'-Hydroxy-5,6,7-trimethoxyflavanone
Catalog No.:BCN0508
CAS No.:72943-91-0
Molecular Network-Guided Alkaloid Profiling of Aerial Parts of Papaver nudicaule L. Using LC-HRMS.[Pubmed:32517053]
Molecules. 2020 Jun 5;25(11). pii: molecules25112636.
Papaver nudicaule L. (Iceland poppy) is widely used for ornamental purposes. A previous study demonstrated the alleviation of lipopolysaccharide-induced inflammation mediated by P. nudicaule extract through nuclear factor-kappa B and signal transducer and activator of transcription 3 inactivation. As isoquinoline alkaloids are chemical markers and bioactive constituents of Papaver species, the present study investigated the alkaloid profile of aerial parts of five P. nudicaule cultivars with different flower colors and a P. rhoeas cropped for two years. A combination of liquid chromatography high-resolution mass spectrometry and molecular networking was used to cluster isoquinoline alkaloids in the species and highlight the possible metabolites. Aside from the 12 compounds, including rotundine, Muramine, and allocryptopine, identified from Global Natural Products Social library and reported information, 46 structurally related metabolites were quantitatively investigated. Forty-two and 16 compounds were proposed for chemical profiles of P. nudicaule and P. rhoeas, respectively. Some species-specific metabolites showed similar fragmentation patterns. The alkaloid abundance of P. nudicaule differed depending on the flower color, and the possible chemical markers were proposed. These results show that molecular networking-guided dereplication allows investigation of unidentified metabolites. The derived chemical profile may facilitate evaluation of P. nudicaule quality for pharmacological applications.
Alkaloids from Corydalis decumbens suppress neuronal excitability in primary cultures of mouse neocortical neurons.[Pubmed:29571149]
Phytochemistry. 2018 Jun;150:85-92.
Eight previously undescribed alkaloids, named corydemine, dihydrocorydemine, corydedine, 8,13-dioxo-14-hydroxytetrahydropalmatine, egenine-alpha-N-oxide, egenine-beta-N-oxide, 7'-O-ethylegenine-alpha-N-oxide, and 7'-O-ethylegenine-beta-N-oxide, together with three known ones, Muramine, l-tetrahydropalmatine, and (+)-egenine, were isolated from the bulbs of Corydalis decumbens. Their structures were elucidated by comprehensive spectroscopic analysis and chemical correlation. The isolated compounds were tested for their ability to modulate neuronal excitability in primary cultured neocortical neurons. Four of the compounds, corydemine, dihydrocorydemine, Muramine, and l-tetrahydropalmatine, inhibited neuronal excitability with IC50 values of 3.6, 16.7, 13.5 and 14.0muM, respectively.
Alkaloids of papaver genus IX. Alkaloids of Glaucium vitellinum Boiss and Buhse, population Seerjan and Glaucium pulchrum Stapf, population Elika.[Pubmed:19671]
Lloydia. 1977 Jul-Aug;40(4):352-5.
Glaucium vitellinum Boiss and Buhse. population Seerjan was shown to contain three major alkaloids, isocorydine (0.44%), protopine (0.42%), dicentrine (0.24%), and four minor alkaloids, tetrahydropalmatine (0.13%), Muramine (0.12%), bulbocapnine (0.06%) and glaucine (0.01%). Glaucium pulchrum Stapf popllation Elika was shown to contain two major alkaloids, corydine (0.3%) and bulbocapnine (0.18%) and three minor alkaloids N-methylindcarpine (0.1%), isocorydine (0.03%) and protopine (0.01%). N-methyllindcarpine was found for the first time in the Papaveraceae and tetrahydropalmatine was detected for the first time in Glaucium.


