SarmentineCAS# 78910-33-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 78910-33-5 | SDF | Download SDF |
| PubChem ID | 6440616 | Appearance | Oil |
| Formula | C14H23NO | M.Wt | 221.3 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
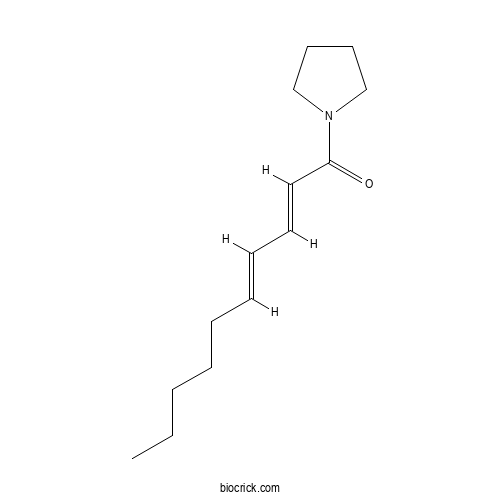
| Chemical Name | (2E,4E)-1-pyrrolidin-1-yldeca-2,4-dien-1-one | ||
| SMILES | CCCCCC=CC=CC(=O)N1CCCC1 | ||
| Standard InChIKey | BFZBGTMIBOQWBA-HRCSPUOPSA-N | ||
| Standard InChI | InChI=1S/C14H23NO/c1-2-3-4-5-6-7-8-11-14(16)15-12-9-10-13-15/h6-8,11H,2-5,9-10,12-13H2,1H3/b7-6+,11-8+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Sarmentine Dilution Calculator

Sarmentine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.5188 mL | 22.5938 mL | 45.1875 mL | 90.3751 mL | 112.9688 mL |
| 5 mM | 0.9038 mL | 4.5188 mL | 9.0375 mL | 18.075 mL | 22.5938 mL |
| 10 mM | 0.4519 mL | 2.2594 mL | 4.5188 mL | 9.0375 mL | 11.2969 mL |
| 50 mM | 0.0904 mL | 0.4519 mL | 0.9038 mL | 1.8075 mL | 2.2594 mL |
| 100 mM | 0.0452 mL | 0.2259 mL | 0.4519 mL | 0.9038 mL | 1.1297 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Muramine
Catalog No.:BCN0496
CAS No.:2292-20-8
- Uvamalol D
Catalog No.:BCN0495
CAS No.:545404-02-2
- 6-O-Veratroylcatalpol
Catalog No.:BCN0494
CAS No.:56973-43-4
- Piceatannol 4'-O-glucoside
Catalog No.:BCN0493
CAS No.:116181-54-5
- Piperchabamide B
Catalog No.:BCN0492
CAS No.:807618-21-9
- Tsaokoin
Catalog No.:BCN0491
CAS No.:343605-41-4
- Apigenin 7,4'-di-O-alloside
Catalog No.:BCN0490
CAS No.:95693-63-3
- 4''-O-Methylcatalposide
Catalog No.:BCN0489
CAS No.:887140-17-2
- Piperdardine
Catalog No.:BCN0488
CAS No.:188426-70-2
- 5-Hydroxy-6,7,3',4'-tetramethoxyflavone
Catalog No.:BCN0487
CAS No.:21763-80-4
- Verproside
Catalog No.:BCN0486
CAS No.:50932-20-2
- Marionol
Catalog No.:BCN0485
CAS No.:65602-55-3
- 5α,8α-Epidioxyergost-6-en-3β-ol
Catalog No.:BCN0498
CAS No.:82227-99-4
- Guineensine
Catalog No.:BCN0499
CAS No.:55038-30-7
- Retrofractamide B
Catalog No.:BCN0500
CAS No.:54794-74-0
- Chingchengenamide A
Catalog No.:BCN0501
CAS No.:139906-29-9
- Isoorientin 2''-O-rhamnoside
Catalog No.:BCN0502
CAS No.:50980-94-4
- 2E,4E-Decadienoylpiperidide
Catalog No.:BCN0503
CAS No.:42997-42-2
- 2E-Decenoylpiperidide
Catalog No.:BCN0504
CAS No.:147030-02-2
- Piperanine
Catalog No.:BCN0505
CAS No.:23512-46-1
- (3R,5R)-1-(4-Hydroxyphenyl)-7-phenylheptane-3,5-diol
Catalog No.:BCN0506
CAS No.:112494-44-7
- Alpinin A
Catalog No.:BCN0507
CAS No.:2151847-03-7
- 4'-Hydroxy-5,6,7-trimethoxyflavanone
Catalog No.:BCN0508
CAS No.:72943-91-0
- 7-O-Methylaromadendrin
Catalog No.:BCN0509
CAS No.:37971-69-0
Interactions Between Natural Herbicides and Lipid Bilayers Mimicking the Plant Plasma Membrane.[Pubmed:30936889]
Front Plant Sci. 2019 Mar 18;10:329.
Natural phytotoxic compounds could become an alternative to traditional herbicides in the framework of sustainable agriculture. Nonanoic acid, Sarmentine and sorgoleone are such molecules extracted from plants and able to inhibit the growth of various plant species. However, their mode of action is not fully understood and despite clues indicating that they could affect the plant plasma membrane, molecular details of such phenomenon are lacking. In this paper, we investigate the interactions between those natural herbicides and artificial bilayers mimicking the plant plasma membrane. First, their ability to affect lipid order and fluidity is evaluated by means of fluorescence measurements. It appears that sorgoleone has a clear ordering effect on lipid bilayers, while nonanoic acid and Sarmentine induce no or little change to these parameters. Then, a thermodynamic characterization of interactions of each compound with lipid vesicles is obtained with isothermal titration calorimetry, and their respective affinity for bilayers is found to be ranked as follows: sorgoleone > Sarmentine > nonanoic acid. Finally, molecular dynamics simulations give molecular details about the location of each compound within a lipid bilayer and confirm the rigidifying effect of sorgoleone. Data also suggest that mismatch in alkyl chain length between nonanoic acid or Sarmentine and lipid hydrophobic tails could be responsible for bilayer destabilization. Results are discussed regarding their implications for the phytotoxicity of these compounds.
[Chemical structure of cyperotundic acid from rhizomes of Cyperus rotundus].[Pubmed:28875671]
Zhongguo Zhong Yao Za Zhi. 2016 Mar;41(6):1066-1069.
Thirteen compounds were isolated from the ethyl acetate extract of the rhizomes of Cyperus rotundus(Xiangfu) by means of various chromatographic techniques(silica gel, Al2O3, Sephadex LH-20, MCI GEL CHP-20P and HPLC), and their structures were identified as cyperotundic acid(1),(4S, 5E, 10R)-7-oxo-trinoreudesm-5-en-4beta-ol(2), 4-hydroxy-4, 7-dimethyl-1-tetralone(3), taraxerone(4), dammaradienyl acetate(5), zeorin(6), Sarmentine(7), guineensine(8), pellitorine(9), caprolactam(10), liriodendrin(11), 3-hydroxy-1-(4-hydroxy-3,5-dimethoxyphenyl)-2-[4-(3-hydroxy-1-(E)-propenyl)-2,6-d imethoxyphenoxy]propyl-beta-D-glucopyranoside(12)and 1-(3, 4-methylenedioxyphenyl)-1E-tetradecene(13) by extensive spectroscopic analyses(IR, MS, 1D-and 2D-NMR). Compound 1 was a new rearranged sesquiterpene and named as cyperotundic acid, which did not obey the isoprene rule.Compounds 2-13 were obtained from the genus Cyperus for the first time.
Sarmentine, a natural herbicide from Piper species with multiple herbicide mechanisms of action.[Pubmed:25904929]
Front Plant Sci. 2015 Apr 8;6:222.
Sarmentine, 1-(1-pyrrolidinyl)-(2E,4E)-2,4-decadien-1-one, is a natural amide isolated from the fruits of Piper species. The compound has a number of interesting biological properties, including its broad-spectrum activity on weeds as a contact herbicide. Initial studies highlighted a similarity in response between plants treated with Sarmentine and herbicidal soaps such as pelargonic acid (nonanoic acid). However, little was known about the mechanism of action leading to the rapid desiccation of foliage treated by Sarmentine. In cucumber cotyledon disc-assays, Sarmentine induced rapid light-independent loss of membrane integrity at 100 muM or higher concentration, whereas 3 mM pelargonic acid was required for a similar effect. Sarmentine was between 10 and 30 times more active than pelargonic acid on wild mustard, velvetleaf, redroot pigweed and crabgrass. Additionally, the potency of 30 muM Sarmentine was greatly stimulated by light, suggesting that this natural product may also interfere with photosynthetic processes. This was confirmed by observing a complete inhibition of photosynthetic electron transport at that concentration. Sarmentine also acted as an inhibitor of photosystem II (PSII) on isolated thylakoid membranes by competing for the binding site of plastoquinone. This can be attributed in part to structural similarities between herbicides like Sarmentine and diuron. While this mechanism of action accounts for the light stimulation of the activity of Sarmentine, it does not account for its ability to destabilize membranes in darkness. In this respect, Sarmentine has some structural similarity to crotonoyl-CoA, the substrate of enoyl-ACP reductase, a key enzyme in the early steps of fatty acid synthesis. Inhibitors of this enzyme, such as triclosan, cause rapid loss of membrane integrity in the dark. Sarmentine inhibited the activity of enoyl-ACP reductase, with an I 50app of 18.3 muM. Therefore, the herbicidal activity of Sarmentine appears to be a complex process associated with multiple mechanisms of action.
Accelerated Stability and Chemical Kinetics of Ethanol Extracts of Fruit of Piper sarmentosum Using High Performance Liquid Chromatography.[Pubmed:24250372]
Iran J Pharm Res. 2011 Summer;10(3):403-13.
The extracts of Piper sarmentosum, a medicinal plant, are being used to prepare phytopharmaceuticals while the information about chemical kinetics of constituents of the extract is unavailable to assign precise shelf life (t90) and find optimum storage conditions of the product for patient safety, and to avoid economic repercussions of launching an unstable product. The extract was exposed to three different conditions of high temperature and relative humidity (RH) for six months. The samples were then analyzed at 0, 1, 2, 4 and 6 months by high performance liquid chromatography (HPLC) using pellitorine, Sarmentine and sarmentosine as markers. Different chemical kinetic parameters of the markers were evaluated by Arrhenius equation to predict shelf life (t90) at different storage conditions and at room temperature. The markers in the extract followed the zero order degradation, and the activation energy, pre exponential factor and rate constant of the reaction of the markers were found to be varying in samples stored at different conditions. The contents of the markers were found to be decreasing at high temperature and humidity with the passage of time. The predicted shelf life (t90) of the markers at room temperature was found to be 16 months approximately. Results of this study indicate that extracts of the plant are stable at room temperature for 16 months. Moreover, the chemical kinetic data of the markers and the analytical method used to quantify the markers may be useful for phytopharmaceutical industry to produce efficacious and stable products from extracts of the plant.
Phytotoxicity of sarmentine isolated from long pepper (Piper longum) fruit.[Pubmed:20839888]
J Agric Food Chem. 2010 Sep 22;58(18):9994-10000.
Discovery of novel natural herbicides has become crucial to overcome increasing weed resistance and environmental issues. In this article, we describe the finding that a methanol extract of dry long pepper (Piper longum L.) fruits is phytotoxic to lettuce (Lactuca sativa L.) seedlings. The bioassay-guided fractionation and purification of the crude extract led to isolation of Sarmentine (1), a known compound, as the active principle. Phytotoxicity of 1 was examined with a variety of seedlings of field crops and weeds. Results indicated that 1 was a contact herbicide and possessed broad-spectrum herbicidal activity. Moreover, a series of Sarmentine analogues were then synthesized to study the structure-activity relationship (SAR). SAR studies suggested that phytotoxicity of Sarmentine and its analogues was specific due to chemical structures, i.e., the analogues of the acid moiety of 1 were active, but the amine and its analogues were inactive; the ester analogues and amide analogues with a primary amine of 1 were also inactive. In addition, quantification of 1 from different resources of the dry P. longum fruits using liquid chromatography-mass spectrometry showed a wide variation, ranging from almost zero to 0.57%. This study suggests that 1 has potential as an active lead molecule for synthesized herbicides as well as for bioherbicides derived from natural resources.
Standardization and in vivo antioxidant activity of ethanol extracts of fruit and leaf of Piper sarmentosum.[Pubmed:19862670]
Planta Med. 2010 Mar;76(5):418-25.
The present study aimed to investigate standardized ethanol extracts of fruit and leaves of Piper sarmentosum for their in vivo antioxidant activity in rats using a CCl (4)-induced oxidative stress model. The standardization was based on the quantification of the markers pellitorine, Sarmentine and sarmentosine by high performance liquid chromatography (HPLC), and determination of total primary and secondary metabolites. The rats, divided into 7 groups each (n = 6), were used as follows: group 1 (CCl (4), negative control), group 2 (untreated, control), groups 3 and 4 (fruit extract 250 and 500 mg/kg, respectively), groups 5 and 6 (leaf extract 250 and 500 mg/kg, respectively) and group 7 (vitamin-E 100 mg/kg, positive control). The doses were administered orally for 14 days; 4 h following the last dose, a single dose of CCl (4) (1.5 mg/kg) was given orally to all the groups except group 2, and after 24 h, blood and liver of each animal were obtained. Analysis of plasma and liver homogenate exhibited significant preservation of markers of antioxidant activity, total plasma antioxidant activity (TPAA), total protein (TP), superoxide dismutase (SOD), catalase (CAT), and thiobarbituric acid reactive species (TBARS), in the pretreated groups as compared to the CCl (4) group (p < 0.05). Histology of the liver also evidenced the protection of hepatocytes against CCl (4) metabolites in the pretreated groups. The results of this study indicate the IN VIVO antioxidant activity of both extracts of the plant, which may be valuable to combat diseases involving free radicals.
Bioactive Markers Based Pharmacokinetic Evaluation of Extracts of a Traditional Medicinal Plant, Piper sarmentosum.[Pubmed:19770264]
Evid Based Complement Alternat Med. 2011;2011:980760.
In vitro assays are economical and easy to perform but to establish relevance of their results to real clinical outcome in animals or human, pharmacokinetics is prerequisite. Despite various in vitro pharmacological activities of extracts of Piper sarmentosum, there is no report of pharmacokinetics. Therefore, the present study aimed to evaluate ethanol extract of fruit of the plant in dose of 500 mg kg(-1) orally for pharmacokinetics. Sprague-Dawley rats were randomly divided into groups 1, 2, and 3 (each n = 6) to study absorption, distribution and excretion, respectively. High performance liquid chromatography (HPLC) with ultraviolet detection was applied to quantify pellitorine, Sarmentine and sarmentosine in plasma, tissues, feces and urine to calculate pharmacokinetic parameters. Pellitorine exhibited maximum plasma concentration (C(max)) 34.77 ng mL(-1) +/- 1.040, time to achieve C(max) (T(max)) 8 h, mean resident time (MRT) 26.00 +/- 0.149 h and half life (t(1/2)) 18.64 +/- 1.65 h. Sarmentine showed C(max) 191.50 +/- 12.69 ng mL(-1), T(max) 6 h, MRT 11.12 +/- 0.44 h and t(1/2) 10.30 +/- 1.98 h. Sarmentosine exhibited zero oral bioavailability because it was neither detected in plasma nor in tissues, and in urine. Pellitorine was found to be distributed in intestinal wall, liver, lungs, kidney, and heart, whereas Sarmentine was found only in intestinal wall and heart. The cumulative excretion of pellitorine, Sarmentine and sarmentosine in feces in 72 h was 0.0773, 0.976, and 0.438 mug, respectively. This study shows that pellitorine and Sarmentine have good oral bioavailability while sarmentosine is not absorbed from the gastrointestinal tract.
Isolation and identification of antiplatelet aggregatory principles from the leaves of Piper lolot.[Pubmed:17941696]
J Agric Food Chem. 2007 Nov 14;55(23):9436-42.
The methanolic extract of Piper lolot, having shown potent inhibitory activity on platelet aggregation induced by arachidonic acid (AA) and platelet activating factor (PAF), was subjected to activity-guided isolation to yield twelve new amide alkaloids, piperlotine A-L (1-12), along with twenty-nine known compounds. Their structures were elucidated on the basis of spectroscopic analysis. The isolated compounds were tested for their inhibitory activity on the rabbit platelet aggregation. The compounds piperlotine A (1), piperlotine C (3), piperlotine D (4), piperlotine E (5), 3-phenyl-1-(2,4,6-trihydroxyphenyl)propan-1-one (21), 3-(4-methoxyphenyl)-1-(2,4,6-trihydroxyphenyl)propan-1-one (22), 1-trans-cinnamoylpyrrolidine (24), Sarmentine (26), pellitorine (27), methyl 3-phenylpropionate (32), and (10S)-10-hydroxypheophorbide a methyl ester (40) showed potent antiplatelet aggregation activity.
In vivo skin antioxidant effect of a new combination based on a specific Vitis vinifera shoot extract and a biotechnological extract.[Pubmed:17691204]
J Drugs Dermatol. 2007 Jun;6(6 Suppl):s8-13.
BACKGROUND AND OBJECTIVES: Ultraviolet (UV) light produces reactive oxygen species (ROS) in skin, which accelerate aging by damaging DNA, proteins, lipids, and other cellular constituents. The aims of this study were to 1) evaluate the antioxidant properties of a Vitis vinifera shoot extract on cultured normal human keratinocytes, 2) compare the in vivo antioxidant of this extract in combination with a biotechnological extract (Ronacare Hydroine), and 3) evaluate the efficacy on photoaging skin of a serum based on a combination (Vitis vinifera shoot extract in hydroglycolic solution, or Sarmentine, and Ronacare Hydroine) after a 4-week application, and to quantify the additional improvement given by applying a cream with the serum. METHODS/STUDY DESIGN: An in vitro study was conducted to evaluate the antioxidant properties of Vitis vinifera shoot extract added to cultured normal human keratinocytes. A fluorescent probe was used to quantify cytoplasmic endogenous species formed in response to oxidative stress induced by H2O2. The antioxidant activity of Vitis vinifera shoot extract was compared to that of a solvent control and 2 positive controls, vitamin E and vitamin C. In the first in vivo study, 2 test products were included in a comparative, randomized, single-blind trial in which 27 subjects acted as their own (untreated) controls. Products were applied 4 times to randomized areas of the inner surface of the forearm for one day. The following day, treated and untreated (control) areas of stratum corneum were sampled for fluorimetric analysis. A decrease in fluorescence compared with untreated control reflected a decrease in the level of ROS, in which case the product had a scavenging effect. The 2 products contained a combination of Sarmentine and Ronacare Hydroine, whose antioxidant properties were under investigation. Other products were known antioxidants. In the second in vivo study, 60 female subjects applied either serum or serum plus cream twice daily for 28 days for clinical evaluation. Overall improvement was rated on a quartile scale (0%-25%, 26%-50%, 51%-75%, 76%-100%) and changes in firmness, radiant glow, evenness, smoothness, wrinkles, fine lines, hydration, texture, and softness were rated on a negative to positive scale (-5=worse to +5=greatly improved). RESULTS AND CONCLUSIONS: Vitis vinifera shoot extract appears to have significantly stronger in vitro antioxidant capacity than vitamin C or vitamin E. In the same vehicle (placebo emulsion), ascorbic acid (0.5%), Sarmentine (1%), and the Sarmentine (1%) plus Ronacare Hydroine (1%) combination had a significant in vivo antioxidant effect versus a nontreated area. The combination Sarmentine (1%) plus Ronacare Hydroine (1%) showed a higher efficacy than Sarmentine alone. The dermatologic evaluation showed that a 4-week twice-daily application of a serum containing the combination improved the main clinical signs of photoaged skin. The addition of the cream with the serum appears to enhance the serum-induced improvement of most of the skin characteristics.
Chemical constituents of the roots of Piper sarmentosum.[Pubmed:16462055]
Chem Pharm Bull (Tokyo). 2006 Feb;54(2):149-51.
Sixteen compounds were isolated from the fresh roots of Piper sarmentosum. Seven of these have been previously isolated from the fruits and leaves of this plant: the aromatic alkene (1), 1-allyl-2-methoxy-4,5-methylenedioxybenzene (4), beta-sitosterol, pyrrole amide (6), Sarmentine (10), sarmentosine (13) and pellitorine (14). (+)-Sesamin (2), horsfieldin (3), two pyrrolidine amides 11 and 12, guineensine (15) and brachystamide B (16) are new for P. sarmentosum. Sarmentamide A, B, and C (7-9) are new natural products. Compounds 1--4 and 6--16 were tested for antiplasmodial, antimycobacterial and antifungal activities.
Chemical constituents and bioactivity of Piper sarmentosum.[Pubmed:15234750]
J Ethnopharmacol. 2004 Aug;93(2-3):173-6.
Eight amides, pellitorine (1), guineensine (2), brachystamide B (3), Sarmentine (4), brachyamide B (5), 1-piperettyl pyrrolidine (6), 3',4',5'-trimethoxycinnamoyl pyrrolidine (7) and sarmentosine (8), two lignans, (+)-asarinin (9) and sesamin (10), and four other compounds, 1-(3,4-methylenedioxyphenyl)-1E-tetradecene (11), methyl piperate (12) and a mixture of beta-sitosterol (13) and stigmasterol (14), were isolated from the fruits of Piper sarmentosum (Piperaceae). This is the first reported isolation of compounds 2, 3, 5, 6, 7, 9, 10 and 12 from this plant species. Their structures were established from spectral data. These compounds were evaluated in antituberculosis and antiplasmodial tests. The results showed that compounds 4 and 6 exhibited both activities while compounds 1, 2, 5, 8 and 11 showed only antituberculosis activity. This is the first report of the antituberculosis and antiplasmodial activities for these compounds.
Cytotoxic amides from Piper sintenense.[Pubmed:12451487]
Planta Med. 2002 Nov;68(11):980-5.
A new alkaloid, pipersintenamide ( 1), together with fourteen known compounds, have been isolated from the whole plant of Piper sintenense. The structures of these compounds were elucidated by spectroscopic analysis. Pipersintenamide, sintenpyridone, Sarmentine, and 1-(3,4-methylenedioxyphenyl)-1 E-dodecene at 20 microg/ml exhibited effective cytotoxicities (cell survival < 15 %) against CCRF-CEM, HL-60, PC-3, and HA22T cell lines.
Constituents of Chinese Piper species and their inhibitory activity on prostaglandin and leukotriene biosynthesis in vitro.[Pubmed:11297843]
J Ethnopharmacol. 2001 May;75(2-3):133-9.
The n-hexane extracts of 19 Piper species, predominantly from China, were screened for their 5-lipoxygenase (5-LOX) and cyclooxygenase-1 (COX-1) inhibitory potential. Many of them showed considerable inhibitory activity against at least one of these two key enzymes of the arachidonic acid metabolism, especially against COX-1. The best results in inhibiting the formation of leukotrienes were obtained with the extract of Piper kadsura. In the terms of prostaglandin synthesis inhibition, the extract of Piper boehmeriifolium var. tonkinense was found to have the strongest activity. Furthermore, an analytical investigation by means of TLC, HPLC-DAD and GC-MS resulted in the identification of 20 constituents. Most of them were amides with an interesting variety of amine moieties. Among them were pellitorine, and four higher homologues, piperlonguminine, dihydropiperlonguminine, futoamide, chingchengenamide, the retrofractamides A, B and D, guineensine, brachystamide B, piperanine, piperine, piperdardine, Sarmentine, pipataline and benzylbenzoate. In 96 cases, these constituents were new for the particular plant.


