GuineensineCAS# 55038-30-7 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 55038-30-7 | SDF | Download SDF |
| PubChem ID | 6442405 | Appearance | Powder |
| Formula | C24H33NO3 | M.Wt | 383.5 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
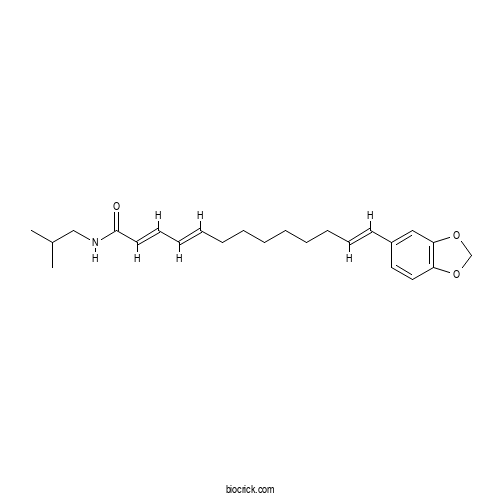
| Chemical Name | (2E,4E,12E)-13-(1,3-benzodioxol-5-yl)-N-(2-methylpropyl)trideca-2,4,12-trienamide | ||
| SMILES | CC(C)CNC(=O)C=CC=CCCCCCCC=CC1=CC2=C(C=C1)OCO2 | ||
| Standard InChIKey | FPMPOFBEYSSYDQ-AUVZEZIHSA-N | ||
| Standard InChI | InChI=1S/C24H33NO3/c1-20(2)18-25-24(26)14-12-10-8-6-4-3-5-7-9-11-13-21-15-16-22-23(17-21)28-19-27-22/h8,10-17,20H,3-7,9,18-19H2,1-2H3,(H,25,26)/b10-8+,13-11+,14-12+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Guineensine Dilution Calculator

Guineensine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6076 mL | 13.0378 mL | 26.0756 mL | 52.1512 mL | 65.189 mL |
| 5 mM | 0.5215 mL | 2.6076 mL | 5.2151 mL | 10.4302 mL | 13.0378 mL |
| 10 mM | 0.2608 mL | 1.3038 mL | 2.6076 mL | 5.2151 mL | 6.5189 mL |
| 50 mM | 0.0522 mL | 0.2608 mL | 0.5215 mL | 1.043 mL | 1.3038 mL |
| 100 mM | 0.0261 mL | 0.1304 mL | 0.2608 mL | 0.5215 mL | 0.6519 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 5α,8α-Epidioxyergost-6-en-3β-ol
Catalog No.:BCN0498
CAS No.:82227-99-4
- Sarmentine
Catalog No.:BCN0497
CAS No.:78910-33-5
- Muramine
Catalog No.:BCN0496
CAS No.:2292-20-8
- Uvamalol D
Catalog No.:BCN0495
CAS No.:545404-02-2
- 6-O-Veratroylcatalpol
Catalog No.:BCN0494
CAS No.:56973-43-4
- Piceatannol 4'-O-glucoside
Catalog No.:BCN0493
CAS No.:116181-54-5
- Piperchabamide B
Catalog No.:BCN0492
CAS No.:807618-21-9
- Tsaokoin
Catalog No.:BCN0491
CAS No.:343605-41-4
- Apigenin 7,4'-di-O-alloside
Catalog No.:BCN0490
CAS No.:95693-63-3
- 4''-O-Methylcatalposide
Catalog No.:BCN0489
CAS No.:887140-17-2
- Piperdardine
Catalog No.:BCN0488
CAS No.:188426-70-2
- 5-Hydroxy-6,7,3',4'-tetramethoxyflavone
Catalog No.:BCN0487
CAS No.:21763-80-4
- Retrofractamide B
Catalog No.:BCN0500
CAS No.:54794-74-0
- Chingchengenamide A
Catalog No.:BCN0501
CAS No.:139906-29-9
- Isoorientin 2''-O-rhamnoside
Catalog No.:BCN0502
CAS No.:50980-94-4
- 2E,4E-Decadienoylpiperidide
Catalog No.:BCN0503
CAS No.:42997-42-2
- 2E-Decenoylpiperidide
Catalog No.:BCN0504
CAS No.:147030-02-2
- Piperanine
Catalog No.:BCN0505
CAS No.:23512-46-1
- (3R,5R)-1-(4-Hydroxyphenyl)-7-phenylheptane-3,5-diol
Catalog No.:BCN0506
CAS No.:112494-44-7
- Alpinin A
Catalog No.:BCN0507
CAS No.:2151847-03-7
- 4'-Hydroxy-5,6,7-trimethoxyflavanone
Catalog No.:BCN0508
CAS No.:72943-91-0
- 7-O-Methylaromadendrin
Catalog No.:BCN0509
CAS No.:37971-69-0
- 4-Formylphenyl(tetra-O-acetyl)-β-D-glucopyranoside
Catalog No.:BCN0510
CAS No.:31873-42-4
- (3R,5R)-1-(4-Hydroxy-3-methoxyphenyl)-7-(3,4-dihydroxyphenyl)heptane-3,5-diol
Catalog No.:BCN0511
CAS No.:408324-13-0
Amide alkaloids characterization and neuroprotective properties of Piper nigrum L.: A comparative study with fruits, pericarp, stalks and leaves.[Pubmed:34474242]
Food Chem. 2021 Aug 10;368:130832.
Piper nigrum L. is commonly used worldwide and its pericarp, stalks, leaves will be major wastes materials. 42 amide alkaloids were identified in black, white pepper and pericarp by UHPLC-LTQ-Orbitrap HRMS method, followed by 40 constituents in stalks and 36 constituents in leaves. 8 amide alkaloids were reported for the first time in P. nigrum. An ultra-high-performance supercritical fluid chromatography (UHPSFC)-MS method was firstly applied to simultaneously determine 9 characteristic constituents (piperine, piperlonguminine, piperanine, pipercallosine, dehydropipernonaline, pipernonatine, retrofractamide B, pellitorine and Guineensine). The most abundant compound in each extract was piperine with a concentration from 0.10 to 12.37 mg/g of dry weight. The fruits, pericarp and leaves extracts could improve cell viability in 6-OHDA-induced SK-N-SH and SH-SY5Y cells. The results showed the characteristics of amide alkaloids of different parts of P. nigrum and evaluated their neuroprotective activities.
Chemical constituents and antibacterial activity from the stems and leaves of Piper wallichii.[Pubmed:34085561]
J Asian Nat Prod Res. 2021 Jun 4:1-8.
A phytochemical investigation of the stems and leaves of Piper wallichii led to the isolation of two new compounds, an aryl alkanone, piwalkanone (1) and a dioxoaporphine alkaloid, piwallidione (2), together with nine known compounds, a dioxoaporphine alkaloid, cepharadione A (3); two aristolactams, piperolactam A (4) and stigmalactam (5); a piperidine, piperine (6); four isobutylamides, piperlonguminine (7), pellitorine (8), N-isobutyl-2E,4E-octadecadienamide (9), and Guineensine (10); and a tyramine, N-trans-feruloyltyramine (11). Their structures were elucidated on the basis of spectroscopic evidence (IR, (1)H NMR, (13)C NMR and 2 D NMR) and MS. Compounds 2 and 3 showed inhibitory activities against pathogenic bacteria, Bacillus cereus, Bacillus subtilis and Staphylococcus aureus.
LC-HRMS/MS-based phytochemical profiling of Piper spices: Global association of piperamides with endocannabinoid system modulation.[Pubmed:33641990]
Food Res Int. 2021 Mar;141:110123.
The plant genus Piper comprises extensively consumed spice taxa like black pepper (P. nigrum L.) or long pepper (P. longum L.). The chronic dietary use of different Piper spices has been associated with different health benefits, though the molecular mechanisms remain poorly understood. The aim of this work was to perform the liquid-chromatography-high-resolution tandem mass spectrometry (LC-HRMS/MS) profiling and LC-DAD quantification of piperamides in several Piper species and varieties and study their ability to modulate the endocannabinoid system (ECS). LC-HRMS/MS analysis revealed a number of 42 piperamides grouped into six structural classes, with 22 of them, notably piperine, retrofractamide B, Guineensine, piperchabamide C, being also quantified by LC-DAD. The multivariate analysis showed that P. nigrum and P. longum are very similar with respect to their piperamide profile, while the other Piper spices (P. retrofractum, P. guineense, P. cubeba, P. borbonense) might have significantly different metabolite patterns. The results from the biological assays confirmed that Guineensine and total piperamides are strongly correlated with anandamide (AEA) cellular uptake inhibition. While none of the Piper spice extracts showed binding activity at cannabinoid CB1 receptors, some P. nigrum varieties exhibited moderate binding interactions with CB2 receptors. Overall, the analytical profiling enabled global annotations of piperamides associated to cannabimimetic effects in Piper spices.
Total Synthesis of the Endocannabinoid Uptake Inhibitor Guineensine and SAR Studies.[Pubmed:31322825]
ChemMedChem. 2019 Sep 4;14(17):1590-1596.
Guineensine ((2E,4E,12E)-13-(benzo[d][1,3]dioxol-5-yl)-N-isobutyltrideca-2,4,12-trienamide) is a plant-derived natural product that inhibits reuptake of the endocannabinoid anandamide with sub-micromolar potency. We have established a highly efficient total synthesis of Guineensine, which provided the natural product in only five steps from commercially available 3-nonyn-1-ol in 17 % overall yield, relying on the attachment of the benzodioxolyl moiety to the unsaturated fatty acid chain by means of a Suzuki coupling as the key step. Subsequent SAR studies revealed that replacement of the N-isobutyl group in the natural product by various alkyl, arylalkyl, or aryl groups is generally well tolerated, and derivatives could be identified that are slightly more potent anandamide reuptake inhibitors than Guineensine itself. In contrast, modifications of the benzodioxolyl moiety led to decreased activity. Intriguingly, a change in the configuration of the C4=C5 double bond from E to Z was found to be very well tolerated, in spite of the associated change in the overall geometry of the molecule.
An Endocannabinoid Uptake Inhibitor from Black Pepper Exerts Pronounced Anti-Inflammatory Effects in Mice.[Pubmed:28942644]
J Agric Food Chem. 2017 Nov 1;65(43):9435-9442.
Guineensine is a dietary N-isobutylamide widely present in black and long pepper (Piper nigrum and Piper longum) previously shown to inhibit cellular endocannabinoid uptake. Given the role of endocannabinoids in inflammation and pain reduction, here we evaluated Guineensine in mouse models of acute and inflammatory pain and endotoxemia. Significant dose-dependent anti-inflammatory effects (95.6 +/- 3.1% inhibition of inflammatory pain at 2.5 mg/kg ip and 50.0 +/- 15.9% inhibition of edema formation at 5 mg/kg ip) and acute analgesia (66.1 +/- 28.1% inhibition at 5.0 mg/kg ip) were observed. Moreover, Guineensine inhibited proinflammatory cytokine production in endotoxemia. Intriguingly, Guineensine and LPS independently induced catalepsy, but in combination this effect was abolished. Both hypothermia and analgesia were blocked by the CB1 receptor inverse agonist rimonabant, but the pronounced hypolocomotion was CB1 receptor-independent. A subsequent screen of 45 CNS-related receptors, ion channels, and transporters revealed apparent interactions of Guineensine with the dopamine transporter DAT, 5HT2A, and sigma receptors, uncovering its prospective polypharmacology. The described potent pharmacological effects of Guineensine might relate to the reported anti-inflammatory effects of pepper.
[Chemical structure of cyperotundic acid from rhizomes of Cyperus rotundus].[Pubmed:28875671]
Zhongguo Zhong Yao Za Zhi. 2016 Mar;41(6):1066-1069.
Thirteen compounds were isolated from the ethyl acetate extract of the rhizomes of Cyperus rotundus(Xiangfu) by means of various chromatographic techniques(silica gel, Al2O3, Sephadex LH-20, MCI GEL CHP-20P and HPLC), and their structures were identified as cyperotundic acid(1),(4S, 5E, 10R)-7-oxo-trinoreudesm-5-en-4beta-ol(2), 4-hydroxy-4, 7-dimethyl-1-tetralone(3), taraxerone(4), dammaradienyl acetate(5), zeorin(6), sarmentine(7), Guineensine(8), pellitorine(9), caprolactam(10), liriodendrin(11), 3-hydroxy-1-(4-hydroxy-3,5-dimethoxyphenyl)-2-[4-(3-hydroxy-1-(E)-propenyl)-2,6-d imethoxyphenoxy]propyl-beta-D-glucopyranoside(12)and 1-(3, 4-methylenedioxyphenyl)-1E-tetradecene(13) by extensive spectroscopic analyses(IR, MS, 1D-and 2D-NMR). Compound 1 was a new rearranged sesquiterpene and named as cyperotundic acid, which did not obey the isoprene rule.Compounds 2-13 were obtained from the genus Cyperus for the first time.
Qualitative and quantitative analysis of an alkaloid fraction from Piper longum L. using ultra-high performance liquid chromatography-diode array detector-electrospray ionization mass spectrometry.[Pubmed:25746504]
J Pharm Biomed Anal. 2015 May 10;109:28-35.
In a previous research, an alkaloid fraction and 18 alkaloid compounds were prepared from Piper longum L. by series of purification process. In this paper, a qualitative and quantitative analysis method using ultra-high performance liquid chromatography-diode array detector-mass spectrometry (UHPLC-DAD-MS) was developed to evaluate the alkaloid fraction. Qualitative analysis of the alkaloid fraction was firstly completed by UHPLC-DAD method and 18 amide alkaloid compounds were identified. A further qualitative analysis of the alkaloid fraction was accomplished by UHPLC-MS/MS method. Another 25 amide alkaloids were identified according to their characteristic ions and neutral losses. At last, a quantitative method for the alkaloid fraction was established using four marker compounds including piperine, pipernonatine, Guineensine and N-isobutyl-2E,4E-octadecadienamide. After the validation of this method, the contents of above four marker compounds in the alkaloid fraction were 57.5mg/g, 65.6mg/g, 17.7mg/g and 23.9mg/g, respectively. Moreover, the relative response factors of other three compounds to piperine were calculated. A comparative study between external standard quantification and relative response factor quantification proved no remarkable difference. UHPLC-DAD-MS method was demonstrated to be a powerful tool for the characterization of the alkaloid fraction from P. longum L. and the result proved that the quality of alkaloid fraction was efficiently improved after appropriate purification.
Guineensine is a novel inhibitor of endocannabinoid uptake showing cannabimimetic behavioral effects in BALB/c mice.[Pubmed:24412246]
Pharmacol Res. 2014 Feb;80:52-65.
High-content screening led to the identification of the N-isobutylamide Guineensine from Piper nigrum as novel nanomolar inhibitor (EC50=290nM) of cellular uptake of the endocannabinoid anandamide (AEA). Noteworthy, Guineensine did not inhibit endocannabinoid degrading enzymes fatty acid amide hydrolase (FAAH) or monoacylglycerol lipase (MAGL) nor interact with cannabinoid receptors or fatty acid binding protein 5 (FABP5), a major cytoplasmic AEA carrier. Activity-based protein profiling showed no inhibition of serine hydrolases. Guineensine also inhibited the cellular uptake of 2-arachidonoylglycerol (2-AG). Preliminary structure-activity relationships between natural Guineensine analogs indicate the importance of the alkyl chain length interconnecting the pharmacophoric isobutylamide and benzodioxol moieties for AEA cellular uptake inhibition. Guineensine dose-dependently induced cannabimimetic effects in BALB/c mice shown by strong catalepsy, hypothermia, reduced locomotion and analgesia. The catalepsy and analgesia were blocked by the CB1 receptor antagonist rimonabant (SR141716A). Guineensine is a novel plant natural product which specifically inhibits endocannabinoid uptake in different cell lines independent of FAAH. Its scaffold may be useful to identify yet unknown targets involved in endocannabinoid transport.
Drug design for neuropathic pain regulation from traditional Chinese medicine.[Pubmed:23378894]
Sci Rep. 2013;3:844.
FAAH-like anandamide transporter (FLAT) regulates anandamide transport for hydrolysis and may be an attractive drug target for pain regulation. We aimed to discover potential FLAT antagonists from traditional Chinese medicine (TCM) using virtual screening, ligand-based drug design and molecular dynamics simulation (MD). Guineensine and Retrofractamide A exhibited high Dock Scores in FLAT. Consensus from multiple linear regression (MLR; R(2) = 08973) and support vector machine (SVM; R(2) = 0.7988) showed similar bioactivities for Guineensine and the FAAH-1 inhibitor (9Z)-1-(5-pyridin-2-yl-1,3,4-oxadiazol-2-yl)octadec-9-en-1-one. Contour of Guineensine to CoMFA and CoMSIA features also imply bioactivity. MD revealed shake or vibration in the secondary structure of FLAT complexed with Guineensine and (9Z)-1-(5-pyridin-2-yl-1,3,4-oxadiazol-2-yl)octadec-9-en-1-one. Ligand movement might contribute to protein changes leading to vibration patterns. Violent vibrations leading to an overall decrease in FLAT function could be the underlying mechanism for Guineensine. Here we suggest Guineensine as a drug-like compound with potential application in relieving neuropathic pain by inhibiting FLAT.
A new conjugated amide-dimer from the aerial parts of Piper submultinerve.[Pubmed:22117113]
Nat Prod Res. 2012;26(19):1824-30.
Bioassay-guided fractionation and purification of the aerial parts of Piper submultinerve led to the isolation of a new conjugated amide-dimer, submultinamide A (1), along with 11 known compounds. The structures were determined on the basis of spectroscopic methods. Among the tested compounds, pellitorine (2), Guineensine (4), N-benzylcinnamide (6) and aristolactam BII (8) showed significant activities in the anti-syncytium assay using (DeltaTat/Rev)MC99 virus and 1A2 cell line system, whereas 2 was most active (EC(5)(0) 35.1 microM and selectivity index 4.7). In the HIV-1 reverse transcriptase assay, only 4 was active with IC(5)(0) 50.8 microM.
Insecticidal activity of isobutylamides derived from Piper nigrum against adult of two mosquito species, Culex pipiens pallens and Aedes aegypti.[Pubmed:22010905]
Nat Prod Res. 2012;26(22):2129-31.
The insecticidal activity of Piper nigrum fruit-derived piperidine alkaloid (piperine) and N-isobutylamide alkaloids (pellitorine, Guineensine, pipercide and retrofractamide A) against female adults of Culex pipiens pallens and Aedes aegypti was examined. On the basis of 24-h LD(50) values, the compound most toxic to female C. pipiens pallens was pellitorine (0.4 microg/female symbol) followed by Guineensine (1.9 microg/female symbol), retrofractamide A (2.4 microg/female symbol) and pipercide (3.2 microg/female symbol). LD(50) value of chlorpyrifos was 0.03 microg/female symbol. Against female A. aegypti, the insecticidal activity was more pronounced in pellitorine (0.17 microg/female symbol) than in retrofractamide A (1.5 microg/female symbol), Guineensine (1.7 microg/female symbol), and pipercide (2.0 microg/female symbol). LD(50) value of chlorpyrifos was 0.0014 microg/female symbol.
Simultaneous determination of bioactive compounds in Piper nigrum L. and a species comparison study using HPLC-PDA.[Pubmed:21854175]
Nat Prod Res. 2011 Aug;25(13):1288-94.
Piper nigrum L. is a traditional medicine widely used in India for illnesses such as constipation, diarrhoea, earache, gangrene, heart disease, hernia, hoarseness, indigestion, insect bites, insomnia, joint pain, liver problems, lung disease, oral abscesses, sunburn, tooth decay and toothaches. In this study, six bioactive compounds, namely piperine (1), pellitorine (2), Guineensine (3), pipnoohine (4), trichostachine (5) and piperonal (6) were quantified in different extracts of P. nigrum L. and compared with those of P. longum L. and P. chaba Hunter. To evaluate the quality of P. nigrum, a simple, accurate and precise HPLC-PDA method was developed for the simultaneous determination of the above-mentioned six compounds. The separation was achieved by Phenomenex Luna RP C(18) column (150 x 4.6 mm, 5 microm, Phenomenex Inc, CA, USA) with a binary gradient solvent system of water-acetonitrile, at a flow rate of 1.0 mL min(-1) and detected at 210, 232, 262 and 343 nm. All six calibration curves showed good linearity (R (2) > 0.9966). The method was reproducible with intra- and inter-day variations of less than 2% and 5%, respectively. The results demonstrated that this method is simple, reliable and suitable for the quality control of these plants.
Practical and efficient approach to the synthesis of guineensine.[Pubmed:21279876]
J Asian Nat Prod Res. 2011 Feb;13(2):128-35.
A total synthesis of Guineensine, a secondary metabolite of the Piperaceae family, has been executed in 12 steps with an overall yield of 27%. Key steps in the synthesis featured novel application of a Julia-Kocienski olefination reaction which effectively constructed alkenamide skeleton. This contributes a unique approach to the synthesis of the piperamide alkaloids.
[Alkaloids and lignans from stems of Piper betle].[Pubmed:21137339]
Zhongguo Zhong Yao Za Zhi. 2010 Sep;35(17):2285-8.
Alkaloids and lignans from the stems of Piper betle were studied. Compounds were isolated and purified by repeated silica gel, reverse phase silica gel, Sephadex LH-20 column chromatography and preparative thin layer chromatography. The structures were elucidated on the basis of spectral analysis. From the ethyl acetate soluble fractions of the 70% acetone extract, ten compounds were isolated and identified as piperine (1), pellitorine (2), N-isobutyl-2E,4E-dodecadienamide (3), dehydropipernonaline (4), piperdardine (5), piperolein-B (6), Guineensine (7), (2E,4E)-N-isobutyl-7-(3',4'-methylenedioxyphenyl)-2,4-heptadienamide (8), syringaresinol-O-beta-D-glucopyranoside (9),pinoresinol (10). All Compounds were isolated from the plant for the first time, and compounds 9 and 10 were isolated firstly from the genus.


