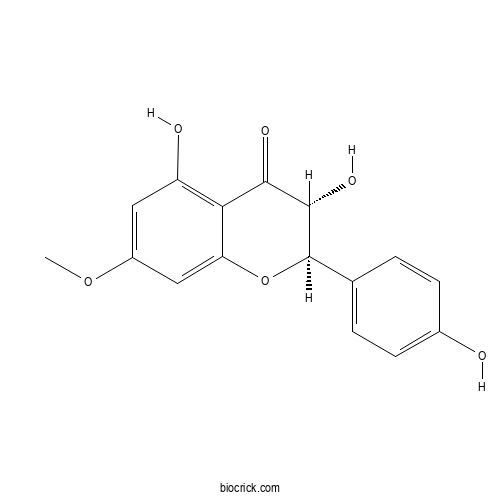
7-O-MethylaromadendrinCAS# 37971-69-0 |
Quality Control & MSDS
Package In Stock
Number of papers citing our products

| Cas No. | 37971-69-0 | SDF | Download SDF |
| PubChem ID | N/A | Appearance | Powder |
| Formula | C16H14O6 | M.Wt | 302.3 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Synonyms | Aromadendrin 7-methyl ether | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

7-O-Methylaromadendrin Dilution Calculator

7-O-Methylaromadendrin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.308 mL | 16.5399 mL | 33.0797 mL | 66.1594 mL | 82.6993 mL |
| 5 mM | 0.6616 mL | 3.308 mL | 6.6159 mL | 13.2319 mL | 16.5399 mL |
| 10 mM | 0.3308 mL | 1.654 mL | 3.308 mL | 6.6159 mL | 8.2699 mL |
| 50 mM | 0.0662 mL | 0.3308 mL | 0.6616 mL | 1.3232 mL | 1.654 mL |
| 100 mM | 0.0331 mL | 0.1654 mL | 0.3308 mL | 0.6616 mL | 0.827 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 4'-Hydroxy-5,6,7-trimethoxyflavanone
Catalog No.:BCN0508
CAS No.:72943-91-0
- Alpinin A
Catalog No.:BCN0507
CAS No.:2151847-03-7
- (3R,5R)-1-(4-Hydroxyphenyl)-7-phenylheptane-3,5-diol
Catalog No.:BCN0506
CAS No.:112494-44-7
- Piperanine
Catalog No.:BCN0505
CAS No.:23512-46-1
- 2E-Decenoylpiperidide
Catalog No.:BCN0504
CAS No.:147030-02-2
- 2E,4E-Decadienoylpiperidide
Catalog No.:BCN0503
CAS No.:42997-42-2
- Isoorientin 2''-O-rhamnoside
Catalog No.:BCN0502
CAS No.:50980-94-4
- Chingchengenamide A
Catalog No.:BCN0501
CAS No.:139906-29-9
- Retrofractamide B
Catalog No.:BCN0500
CAS No.:54794-74-0
- Guineensine
Catalog No.:BCN0499
CAS No.:55038-30-7
- 5α,8α-Epidioxyergost-6-en-3β-ol
Catalog No.:BCN0498
CAS No.:82227-99-4
- Sarmentine
Catalog No.:BCN0497
CAS No.:78910-33-5
- 4-Formylphenyl(tetra-O-acetyl)-β-D-glucopyranoside
Catalog No.:BCN0510
CAS No.:31873-42-4
- (3R,5R)-1-(4-Hydroxy-3-methoxyphenyl)-7-(3,4-dihydroxyphenyl)heptane-3,5-diol
Catalog No.:BCN0511
CAS No.:408324-13-0
- Methyl epi-dihydrophaseate
Catalog No.:BCN0512
CAS No.:57761-30-5
- Linarin 4'''-acetate
Catalog No.:BCN0513
CAS No.:79541-06-3
- Myricetin 3-O-beta-D-xylopyranosyl(1-2)-[alpha-L-rhamnopyranosyl-(1-6)]-beta-D-glucopyranoside
Catalog No.:BCN0514
CAS No.:
- Alpinin B
Catalog No.:BCN0515
CAS No.:2125947-85-3
- (R)-5-Hydroxy-7-(4-hydroxyphenyl)-1-phenylheptan-3-one
Catalog No.:BCN0516
CAS No.:1961196-24-6
- Desacetylripariochromene B
Catalog No.:BCN0517
CAS No.:69790-24-5
- Grandiuvarin A
Catalog No.:BCN0518
CAS No.:882692-93-5
- 5,7,8-Trimethoxyflavanone
Catalog No.:BCN0519
CAS No.:69616-73-5
- 5,6,7,4'-Tetramethoxyflavanone
Catalog No.:BCN0520
CAS No.:72943-90-9
- 2'-Hydroxy-3',4',6'-trimethoxydihydrochalcone
Catalog No.:BCN0521
CAS No.:1222818-87-2
Phytochemical, Antiplasmodial, Cytotoxic and Antimicrobial Evaluation of a Southeast Brazilian Brown Propolis Produced by Apis mellifera Bees.[Pubmed:34227213]
Chem Biodivers. 2021 Sep;18(9):e2100288.
Seven phenolic compounds (ferulic acid, caffeic acid, 4-methoxycinnamic acid, 3,4-dimethoxycinnamic acid, 3-hydroxy-4-methoxybenzaldehyde, 3-methoxy-4-hydroxypropiophenone and 1-O,2-O-digalloyl-6-O-trans-p-coumaroyl-beta-D-glucopyranoside), a flavanonol (7-O-Methylaromadendrin), two lignans (pinoresinol and matairesinol) and six diterpenic acids/alcohol (19-acetoxy-13-hydroxyabda-8(17),14-diene, totarol, 7-oxodehydroabietic acid, dehydroabietic acid, communic acid and isopimaric acid) were isolated from the hydroalcoholic extract of a Brazilian Brown Propolis and characterized by NMR spectral data analysis. The volatile fraction of brown propolis was characterized by CG-MS, composed mainly of monoterpenes and sesquiterpenes, being the major alpha-pinene (18.4 %) and beta-pinene (10.3 %). This propolis chemical profile indicates that Pinus spp., Eucalyptus spp. and Araucaria angustifolia might be its primary plants source. The brown propolis displayed significant activity against Plasmodium falciparum D6 and W2 strains with IC50 of 5.3 and 9.7 mug/mL, respectively. The volatile fraction was also active with IC50 of 22.5 and 41.8 mug/mL, respectively. Among the compounds, 1-O,2-O-digalloyl-6-O-trans-p-coumaroyl-beta-D-glucopyranoside showed IC50 of 3.1 and 1.0 mug/mL against D6 and W2 strains, respectively, while communic acid showed an IC50 of 4.0 mug/mL against W2 strain. Cytotoxicity was determined on four tumor cell lines (SK-MEL, KB, BT-549, and SK-OV-3) and two normal renal cell lines (LLC-PK1 and VERO). Matairesinol, 7-O-Methylaromadendrin, and isopimaric acid showed an IC50 range of 1.8-0.78 mug/mL, 7.3-100 mug/mL, and 17-18 mug/mL, respectively, against the tumor cell lines but they were not cytotoxic against normal cell lines. The crude extract of brown propolis displayed antimicrobial activity against C. neoformans, methicillin-resistant Staphylococcus aureus, and P. aeruginosa at 29.9 mug/mL, 178.9 mug/mL, and 160.7 mug/mL, respectively. The volatile fraction inhibited the growth of C. neoformans at 53.0 mug/mL. The compounds 3-hydroxy-4-methoxybenzaldehyde, 3-methoxy-4-hydroxypropiophenone and 7-oxodehydroabietic acid were active against C. neoformans, and caffeic and communic acids were active against methicillin-resistant Staphylococcus aureus.
Alkylating enzymes.[Pubmed:23518239]
Curr Opin Chem Biol. 2013 Apr;17(2):229-35.
Chemospecific and regiospecific modifications of natural products by methyl, prenyl, or C-glycosyl moieties are a challenging and cumbersome task in organic synthesis. Because of the availability of an increasing number of stable and selective transferases and cofactor regeneration processes, enzyme-assisted strategies turn out to be promising alternatives to classical synthesis. Two categories of alkylating enzymes become increasingly relevant for applications: firstly prenyltransferases and terpene synthases (including terpene cyclases), which are used in the production of terpenoids such as artemisinin, or meroterpenoids like alkylated phenolics and indoles, and secondly methyltransferases, which modify flavonoids and alkaloids to yield products with a specific methylation pattern such as 7-O-Methylaromadendrin and scopolamine.
[Chemical constituents contained in Populus tomentosa].[Pubmed:22860454]
Zhongguo Zhong Yao Za Zhi. 2012 May;37(10):1422-5.
OBJECTIVE: To separate and identify chemical constituents from stem barks of male plants of Populus tomentosa. METHOD: Fresh stem barks of P. tomentosa were extracted with methanol to obtain extracts which were suspended in water and blended successively with petroleum ether, ethyl acetate and n-butanol. Various chromatographic techniques were used to separate and purify the constituents extracted with ethyl acetate and n-butanol fractions. Their structures were identified on the basis of their physicochemical properties and spectral data. RESULT: Twelve compounds were separated with ethyl acetate and n-butanol fractions and identified as benzoic acid (1), daucosterol (2), tremuloidin (3), rhamnocitrin (4), sakuranetin (5), 7-O-Methylaromadendrin (6), isograndidentatin A (7), siebolside B (8), sakuranin (9), micranthoside (10), alpha-D-glucopyranose (11), and sucrose (12). CONCLUSION: Compounds 4-12 were separated from this plant for the first time. Of them, compound 10 was separated from this plant genus for the first time.
7-O-methylaromadendrin stimulates glucose uptake and improves insulin resistance in vitro.[Pubmed:20823563]
Biol Pharm Bull. 2010;33(9):1494-9.
The stimulation of glucose uptake into peripheral tissues is an important mechanism for the removal of glucose in blood and for the management of diabetes mellitus (DM). Since recent results have demonstrated the beneficial effects of flavonoids in relation to DM, this study was designed to examine the effects of 7-O-Methylaromadendrin (7-O-MA), a flavonoid isolated from Inula viscosa, on glucose uptake into liver and fat tissue, and investigate the molecular mechanisms involved. 7-O-MA at 10 microM significantly stimulated insulin-induced glucose uptake measured by 2-[N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino]-2-deoxy-D-glucose (2-NBDG) in both human hepatocellular liver carcinoma (HepG2) cells and differentiated 3T3-L1 adipocytes. Adipocyte-specific fatty acid binding protein (aP2) gene expression was increased by 7-O-MA in adipocytes, and both gene and protein level of peroxisome proliferator-activated receptor gamma2 (PPARgamma2) was also increased. Moreover, 7-O-MA stimulated the reactivation of insulin-mediated phosphorylation of phosphatidylinositol 3-kinase (PI3K)-linked protein kinase B (Akt/PKB) and adenosine 5'-monophosphate-activated protein kinase (AMPK) in high glucose-induced, insulin-resistant HepG2 cells, and this effect was blocked by either LY294002, a PI3K inhibitor, or compound C, an AMPK inhibitor. Therefore, these results suggest that 7-O-MA might stimulate glucose uptake via PPARgamma2 activation and improve insulin resistance via PI3K and AMPK-dependent pathways, and be a potential candidate for the management of type 2 DM.
Cytotoxic effects of compounds from Iris tectorum on human cancer cell lines.[Pubmed:18508214]
J Ethnopharmacol. 2008 Jul 23;118(2):257-63.
In the course of searching for novel cytotoxic compounds which can be used in chemotherapy, several Traditional Chinese Medicines (TCM) have been screened by bioassay-guided fractionation and isolation. An extract of rhizomes of Iris tectorum Maxim., a TCM used to treat cancer, exhibited highest potency and led to the isolation of two flavonoids, 7-O-Methylaromadendrin and tectorigenin, and four iridal-type triterpenes, iritectols A and B, isoiridogermanal and iridobelamal A. The cytotoxicities of the isolated compounds against four human cancer cell lines were evaluated by the SRB assay. Iritectol B, isoiridogermanal and iridobelamal A showed similar cytotoxicity with IG(50) around 11 microM and 23 microM against MCF-7 and C32 cell lines, respectively. Cell cycle-specific inhibition and apoptosis induced by the isolated compounds were determined using flow cytometry with two sets of co-labelling systems: annexin V-FITC/propidium iodide and fluorescein diacetate/propidium iodide. Iritectol B demonstrated dose-dependent apoptotic effect against COR-L23 cells with an apoptotic rate of 33% at 100 microM. Tectorigenin (an analogue of genistein) showed cell cycle specific inhibition and arrested cells at G(2)/M phase up to 400 microM, but did not demonstrate apoptotic effect against COR-L23 cells up to 1 mM. The overall activities of isolated compounds observed in the present study support the traditional use of Iris tectorum Maxim. in the treatment of cancer.
Effects of naturally occurring dihydroflavonols from Inula viscosa on inflammation and enzymes involved in the arachidonic acid metabolism.[Pubmed:17658557]
Life Sci. 2007 Jul 19;81(6):480-8.
The anti-inflammatory properties of three flavanones isolated from Inula viscosa, sakuranetin, 7-O-Methylaromadendrin, and 3-acetyl-7-O-Methylaromadendrin, have been tested both in vitro and in vivo. Acute inflammation in vivo was induced by means of topical application of 12-O-tetradecanoylphorbol 13-acetate (TPA) to mouse ears or by subcutaneous injection of phospholipase A(2) (PLA(2)) into mouse paws. The test compounds were evaluated in vitro for their effect on both the metabolism of arachidonic acid and on the release and/or activity of enzymes involved in the inflammatory response such as elastase, myeloperoxidase (MPO), and protein kinase C (PKC). The most active compounds in vivo against PLA(2)-induced paw oedema were 7-O-Methylaromadendrin (ED(50)=8 mg/kg) and sakuranetin (ED(50)=18 mg/kg). In contrast, the most potent compound against TPA-induced ear oedema was 3-acetyl-7-O-Methylaromadendrin (ED(50)=185 microg/ear), followed by sakuranetin (ED(50)=205 microg/ear). In vitro, the latter compound was the most potent inhibitor of leukotriene (LT) B(4) production by peritoneal rat neutrophils (IC(50)=9 microM) and it was also the only compound that directly inhibited the activity of 5-lipoxygenase (5-LOX). 3-Acetyl-7-O-Methylaromadendrin also inhibited LTB(4) production (IC(50)=15 microM), but had no effect on 5-LOX activity. The only flavanone that inhibited the secretory PLA(2) activity in vitro was 7-O-Methylaromadendrin. This finding may partly explain the anti-inflammatory effect observed in vivo, although other mechanisms such as the inhibition of histamine release by mast cells may also be implicated. Sakuranetin at 100 microM was found to inhibit elastase release, although this result is partly due to direct inhibition of the enzyme itself. At the same concentration, 7-O-Methylaromadendrin only affected the enzyme release. Finally, none of the flavanones exhibited any effect on MPO or PKC activities. Taken together, these findings indicate that sakuranetin may be a selective inhibitor of 5-LOX.
Anti-inflammatory activity of flavonoids from Populus davidiana.[Pubmed:17225458]
Arch Pharm Res. 2006 Dec;29(12):1102-8.
An in vitro bioassay-guide revealed that the methanol (MeOH) extract of the stem bark of Populus davidiana showed considerable inhibitory activity against cyclooxygenase (COX-1, COX-2). Continuous phytochemical study of the MeOH extract of this plant led to the isolation of ten flavonoids; sakuranetin (1), rhamnocitrin (2), 7-O-Methylaromadendrin (3), naringenin (4), eriodictyol (5), aromadendrin (6), kaempferol (7), neosakuranin (8), sakuranin (9) and sakurenetin-5,4'-di-beta-D-glucopyranoside (10). Their structures were identified on the basis of their physicochemical and spectroscopic analyses. The isolated compounds, 1-10, were tested for their inhibitory activities against COX-1 and COX-2. Compound 7 was found to have potent inhibitory effect on COX-1 and a moderate effect on COX-2, meanwhile, compounds 1-6 showed moderate inhibition against COX-1 only. Moreover, compounds 5-8 exhibited suppressive effects on xanthine oxidase (XO). These results may explain, in part, the traditional uses of P. davidiana in ethnomedicine.
A glycosyl analogue of diacylglycerol and other antiinflammatory constituents from Inula viscosa.[Pubmed:10217718]
J Nat Prod. 1999 Apr;62(4):601-4.
Some extracts from Inula viscosa were examined for acute antiinflammatory activity in vivo. Three flavonoids: rhamnocitrin (1), 7-O-Methylaromadendrin (3), and 3-O-acetylpadmatin (4); a sesquiterpene lactone, inuviscolide (2); a sesquiterpene acid, ilicic acid (5); and a digalactosyl-diacylglycerol, inugalactolipid A (6), were isolated from the CH2Cl2 extract, identified by spectroscopic methods, and characterized as the topical antiinflammatory principles of this species. All these compounds proved to be effective against 12-O-tetradecanoylphorbol-13-acetate-induced ear edema in mice, although lacking activity against arachidonic acid-induced edema. In addition, compounds 5 and, markedly, 6 showed notable effects on a multiple-dose murine chronic dermatitis model. This is the first attempt to establish a rationale concerning the documented use of the plant on various skin diseases.


