NBD-557HIV-1 entry inhibitor,block gp120-CD4 interaction CAS# 333352-59-3 |
- Limonin
Catalog No.:BCN6057
CAS No.:1180-71-8
- Fosamprenavir Calcium Salt
Catalog No.:BCC1581
CAS No.:226700-81-8
- HIV-1 integrase inhibitor
Catalog No.:BCC1618
CAS No.:544467-07-4
- BMS-626529
Catalog No.:BCC1427
CAS No.:701213-36-7
- HIV-1 integrase inhibitor 2
Catalog No.:BCC1619
CAS No.:957890-42-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 333352-59-3 | SDF | Download SDF |
| PubChem ID | 5279270 | Appearance | Powder |
| Formula | C17H24BrN3O2 | M.Wt | 382.3 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 10 mg/mL (26.16 mM; Need ultrasonic) | ||
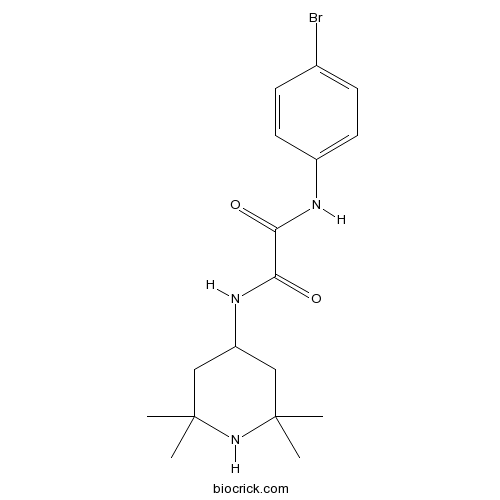
| Chemical Name | N'-(4-bromophenyl)-N-(2,2,6,6-tetramethylpiperidin-4-yl)oxamide | ||
| SMILES | CC1(CC(CC(N1)(C)C)NC(=O)C(=O)NC2=CC=C(C=C2)Br)C | ||
| Standard InChIKey | QQRFLGRIDNNARB-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C17H24BrN3O2/c1-16(2)9-13(10-17(3,4)21-16)20-15(23)14(22)19-12-7-5-11(18)6-8-12/h5-8,13,21H,9-10H2,1-4H3,(H,19,22)(H,20,23) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | NBD-557 is a potentially HIV-1 inhibitor.
IC50 Value:
Target: HIV
NBD-557, is small molecule organic compounds with drug-like properties. It showed potent cell fusion and virus-cell fusion inhibitory activity at low micromolar levels. A systematic study showed that NBD-557 target viral entry by inhibiting the binding of HIV-1 envelope glycoprotein gp120 to the cellular receptor CD4 but did not inhibit reverse transcriptase, integrase, or protease, indicating that they do not target the later stages of the HIV-1 life cycle to inhibit HIV-1 infection. NBD-557 potent inhibitors of both X4 and R5 viruses tested in CXCR4 and CCR5 expressing cell lines, respectively, indicating that its anti-HIV-1 activity is not dependent on the coreceptor tropism of the virus. A surface plasmon resonance study, which measures binding affinity, clearly demonstrated that NBD-557 bind to unliganded HIV-1 gp120 but not to the cellular receptor CD4. NBD-557 was active against HIV-1 laboratory-adapted strains including an AZT-resistant strain and HIV-1 primary isolates, indicating that NBD-557 can potentially be further modified to become potent HIV-1 entry inhibitors. References: | |||||

NBD-557 Dilution Calculator

NBD-557 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6157 mL | 13.0787 mL | 26.1575 mL | 52.3149 mL | 65.3937 mL |
| 5 mM | 0.5231 mL | 2.6157 mL | 5.2315 mL | 10.463 mL | 13.0787 mL |
| 10 mM | 0.2616 mL | 1.3079 mL | 2.6157 mL | 5.2315 mL | 6.5394 mL |
| 50 mM | 0.0523 mL | 0.2616 mL | 0.5231 mL | 1.0463 mL | 1.3079 mL |
| 100 mM | 0.0262 mL | 0.1308 mL | 0.2616 mL | 0.5231 mL | 0.6539 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
NBD-557 is a potentially HIV-1 inhibitor.
- Boc-Phe(4-NO2)-OH
Catalog No.:BCC3275
CAS No.:33305-77-0
- Dipsacoside B
Catalog No.:BCN5940
CAS No.:33289-85-9
- Gummiferin
Catalog No.:BCN8381
CAS No.:33286-30-5
- Diltiazem HCl
Catalog No.:BCC4901
CAS No.:33286-22-5
- 5,6-Dihydroyangonin
Catalog No.:BCN3566
CAS No.:3328-60-7
- 5-Aminofluorescein
Catalog No.:BCC8733
CAS No.:3326-34-9
- ML SA1
Catalog No.:BCC6276
CAS No.:332382-54-4
- Strychnistenolide
Catalog No.:BCN8039
CAS No.:332372-09-5
- 1,7-Bis(4-hydroxyphenyl)hepta-4,6-dien-3-one
Catalog No.:BCN7092
CAS No.:332371-82-1
- Rutaevin
Catalog No.:BCN6993
CAS No.:33237-37-5
- DZ2002
Catalog No.:BCC5544
CAS No.:33231-14-0
- Glucosyringic acid
Catalog No.:BCN5254
CAS No.:33228-65-8
- NBD-556
Catalog No.:BCC1790
CAS No.:333353-44-9
- Trianthenol
Catalog No.:BCN7802
CAS No.:333361-85-6
- Gliquidone
Catalog No.:BCC5003
CAS No.:33342-05-1
- Theaflavine-3,3'-digallate
Catalog No.:BCN5420
CAS No.:33377-72-9
- 9-O-Methyl-4-hydroxyboeravinone B
Catalog No.:BCN4063
CAS No.:333798-10-0
- Z-Asp(OtBu)-OSu
Catalog No.:BCC2787
CAS No.:3338-32-7
- 8-Deoxygartanin
Catalog No.:BCN5255
CAS No.:33390-41-9
- Gartanin
Catalog No.:BCN5256
CAS No.:33390-42-0
- LUF 5834
Catalog No.:BCC6237
CAS No.:333962-91-7
- (E)-2-Decenoic acid
Catalog No.:BCC1292
CAS No.:334-49-6
- Spermidine trihydrochloride
Catalog No.:BCC6865
CAS No.:334-50-9
- 3-Hydroxy-3-acetonyloxindole
Catalog No.:BCN4069
CAS No.:33417-17-3
Identification of N-phenyl-N'-(2,2,6,6-tetramethyl-piperidin-4-yl)-oxalamides as a new class of HIV-1 entry inhibitors that prevent gp120 binding to CD4.[Pubmed:15996703]
Virology. 2005 Sep 1;339(2):213-25.
We have identified two N-phenyl-N'-(2,2,6,6-tetramethyl-piperidin-4-yl)-oxalamide analogs as a novel class of human immunodeficiency virus type 1 (HIV-1) entry inhibitors that block the gp120-CD4 interaction, using database screening techniques. The lead compounds, NBD-556 and NBD-557, are small molecule organic compounds with drug-like properties. These compounds showed potent cell fusion and virus-cell fusion inhibitory activity at low micromolar levels. A systematic study showed that these compounds target viral entry by inhibiting the binding of HIV-1 envelope glycoprotein gp120 to the cellular receptor CD4 but did not inhibit reverse transcriptase, integrase, or protease, indicating that they do not target the later stages of the HIV-1 life cycle to inhibit HIV-1 infection. These compounds were equally potent inhibitors of both X4 and R5 viruses tested in CXCR4 and CCR5 expressing cell lines, respectively, indicating that their anti-HIV-1 activity is not dependent on the coreceptor tropism of the virus. A surface plasmon resonance study, which measures binding affinity, clearly demonstrated that these compounds bind to unliganded HIV-1 gp120 but not to the cellular receptor CD4. NBD-556 and NBD-557 were active against HIV-1 laboratory-adapted strains including an AZT-resistant strain and HIV-1 primary isolates, indicating that these compounds can potentially be further modified to become potent HIV-1 entry inhibitors.
Crystal structures of HIV-1 gp120 envelope glycoprotein in complex with NBD analogues that target the CD4-binding site.[Pubmed:24489681]
PLoS One. 2014 Jan 28;9(1):e85940.
Efforts to develop therapeutic agents that inhibit HIV-1 entry have led to the identification of several small molecule leads. One of the most promising is the NBD series, which binds within a conserved gp120 cavity and possesses para-halogen substituted aromatic rings, a central oxalamide linker, and a tetramethylpiperidine moiety. In this study, we characterized structurally the interactions of four NBD analogues containing meta-fluoro substitution on the aromatic ring and various heterocyclic ring replacements of the tetramethylpiperidine group. The addition of a meta-fluorine to the aromatic ring improved surface complementarity and did not alter the position of the analogue relative to gp120. By contrast, heterocyclic ring replacements of the tetramethylpiperidine moiety exhibited diverse positioning and interactions with the vestibule of the gp120 cavity. Overall, the biological profile of NBD-congeners was modulated by ligand interactions with the gp120-cavity vestibule. Herein, six co-crystal structures of NBD-analogues with gp120 provide a structural framework for continued small molecule-entry inhibitor optimization.
Thermodynamics of binding of a low-molecular-weight CD4 mimetic to HIV-1 gp120.[Pubmed:16953583]
Biochemistry. 2006 Sep 12;45(36):10973-80.
NBD-556 and the chemically and structurally similar NBD-557 are two low-molecular weight compounds that reportedly block the interaction between the HIV-1 envelope glycoprotein gp120 and its receptor, CD4. NBD-556 binds to gp120 with a binding affinity of 2.7 x 10(5) M(-1) (K(d) = 3.7 muM) in a process characterized by a large favorable change in enthalpy partially compensated by a large unfavorable entropy change, a thermodynamic signature similar to that observed for binding of sCD4 to gp120. NBD-556 binding is associated with a large structuring of the gp120 molecule, as also demonstrated by CD spectroscopy. NBD-556, like CD4, activates the binding of gp120 to the HIV-1 coreceptor, CCR5, and to the 17b monoclonal antibody, which recognizes the coreceptor binding site of gp120. NBD-556 stimulates HIV-1 infection of CD4-negative, CCR5-expressing cells. The thermodynamic signature of the binding of NBD-556 to gp120 is very different from that of another viral entry inhibitor, BMS-378806. Whereas NBD-556 binds gp120 with a large favorable enthalpy and compensating unfavorable entropy changes, BMS-378806 does so with a small binding enthalpy change in a mostly entropy-driven process. NBD-556 is a competitive inhibitor of sCD4 and elicits a similar structuring of the coreceptor binding site, whereas BMS-378806 does not compete with sCD4 and does not induce coreceptor binding. These studies demonstrate that low-molecular-weight compounds can induce conformational changes in the HIV-1 gp120 glycoprotein similar to those observed upon CD4 binding, revealing distinct strategies for inhibiting the function of the HIV-1 gp120 envelope glycoprotein. Furthermore, competitive and noncompetitive compounds have characteristic thermodynamic signatures that can be used to guide the design of more potent and effective viral entry inhibitors.
Small molecule HIV entry inhibitors: Part II. Attachment and fusion inhibitors: 2004-2010.[Pubmed:21342055]
Expert Opin Ther Pat. 2011 Mar;21(3):399-416.
INTRODUCTION: The first US FDA approved HIV entry inhibitor drug Enfuvirdine belongs to the fusion inhibitor category. Earlier efforts in this area were focused on peptides and monoclonal antibodies; recently, the focus has shifted towards the development of small molecule HIV attachment and fusion inhibitors. They can be used for prophylactic purposes and also hold potential for the development of HIV microbicides. AREAS COVERED: In a previous paper ('Small molecule HIV entry inhibitors: Part I'), we reviewed patents and patent applications for small molecule chemokine receptor antagonists from major pharmaceutical companies. In this paper, the development of small molecule HIV attachment and fusion inhibitors is discussed in detail. It covers patents and patent applications for small molecule HIV attachment and fusion inhibitors published between 2004 and 2010 and related literature with a focus on recent developments based on lead generation and lead modification. EXPERT OPINION: To augment the potency of currently available antiretroviral drug combinations and to fight drug-resistant virus variants, more effective drugs which target additional steps in the viral replication cycle are urgently needed. HIV attachment and fusion processes are such targets. Inhibitors of these targets will provide additional options for the treatment of HIV drug-resistant strains. Small molecule HIV attachment inhibitors such as BMS-378806 and analogs from Bristol Myers Squibb, N-aryl piperidine derivatives from Propharmacon, and NBD-556 and NBD-557 from New York Blood Center may have potential as vaginal microbicidal agents and can be an economical alternative to monoclonal antibodies.
Binding mode characterization of NBD series CD4-mimetic HIV-1 entry inhibitors by X-ray structure and resistance study.[Pubmed:25001301]
Antimicrob Agents Chemother. 2014 Sep;58(9):5478-91.
We previously identified two small-molecule CD4 mimetics--NBD-556 and NBD-557--and synthesized a series of NBD compounds that resulted in improved neutralization activity in a single-cycle HIV-1 infectivity assay. For the current investigation, we selected several of the most active compounds and assessed their antiviral activity on a panel of 53 reference HIV-1 Env pseudoviruses representing diverse clades of clinical isolates. The selected compounds inhibited tested clades with low-micromolar potencies. Mechanism studies indicated that they act as CD4 agonists, a potentially unfavorable therapeutic trait, in that they can bind to the gp120 envelope glycoprotein and initiate a similar physiological response as CD4. However, one of the compounds, NBD-09027, exhibited reduced agonist properties, in both functional and biophysical studies. To understand the binding mode of these inhibitors, we first generated HIV-1-resistant mutants, assessed their behavior with NBD compounds, and determined the X-ray structures of two inhibitors, NBD-09027 and NBD-10007, in complex with the HIV-1 gp120 core at approximately 2-A resolution. Both studies confirmed that the NBD compounds bind similarly to NBD-556 and NBD-557 by inserting their hydrophobic groups into the Phe43 cavity of gp120. The basic nitrogen of the piperidine ring is located in close proximity to D368 of gp120 but it does not form any H-bond or salt bridge, a likely explanation for their nonoptimal antagonist properties. The results reveal the structural and biological character of the NBD series of CD4 mimetics and identify ways to reduce their agonist properties and convert them to antagonists.


