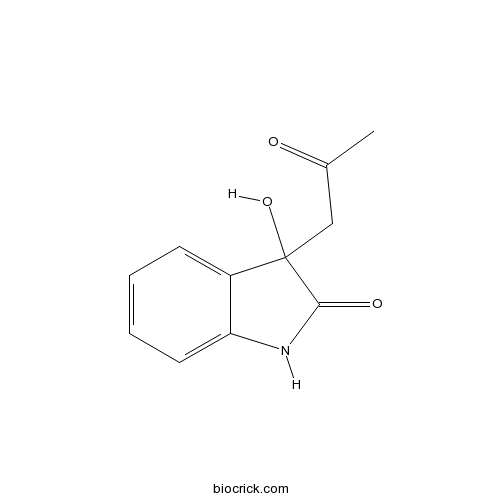
3-Hydroxy-3-acetonyloxindoleCAS# 33417-17-3 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 33417-17-3 | SDF | Download SDF |
| PubChem ID | 36460 | Appearance | Powder |
| Formula | C11H11NO3 | M.Wt | 205.2 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 3-hydroxy-3-(2-oxopropyl)-1H-indol-2-one | ||
| SMILES | CC(=O)CC1(C2=CC=CC=C2NC1=O)O | ||
| Standard InChIKey | CBMTTXBZZZABGG-UHFFFAOYSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Standard reference |
| In vivo | Biological studies on indole derivatives. I. Synthesis and pharmacological studies of 2-oxo-3-indolyl derivatives.[Pubmed: 3604703]Acta Physiol Pharmacol Latinoam. 1986;36(4):391-5.
|
| Structure Identification | J Pharm Sci. 1980 Oct;69(10):1235-7.Synthesis of potential anticonvulsants: condensation of isatins with acetone and related ketones.[Pubmed: 6158569]
|

3-Hydroxy-3-acetonyloxindole Dilution Calculator

3-Hydroxy-3-acetonyloxindole Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.8733 mL | 24.3665 mL | 48.7329 mL | 97.4659 mL | 121.8324 mL |
| 5 mM | 0.9747 mL | 4.8733 mL | 9.7466 mL | 19.4932 mL | 24.3665 mL |
| 10 mM | 0.4873 mL | 2.4366 mL | 4.8733 mL | 9.7466 mL | 12.1832 mL |
| 50 mM | 0.0975 mL | 0.4873 mL | 0.9747 mL | 1.9493 mL | 2.4366 mL |
| 100 mM | 0.0487 mL | 0.2437 mL | 0.4873 mL | 0.9747 mL | 1.2183 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Spermidine trihydrochloride
Catalog No.:BCC6865
CAS No.:334-50-9
- (E)-2-Decenoic acid
Catalog No.:BCC1292
CAS No.:334-49-6
- LUF 5834
Catalog No.:BCC6237
CAS No.:333962-91-7
- Gartanin
Catalog No.:BCN5256
CAS No.:33390-42-0
- 8-Deoxygartanin
Catalog No.:BCN5255
CAS No.:33390-41-9
- Z-Asp(OtBu)-OSu
Catalog No.:BCC2787
CAS No.:3338-32-7
- 9-O-Methyl-4-hydroxyboeravinone B
Catalog No.:BCN4063
CAS No.:333798-10-0
- Theaflavine-3,3'-digallate
Catalog No.:BCN5420
CAS No.:33377-72-9
- Gliquidone
Catalog No.:BCC5003
CAS No.:33342-05-1
- Trianthenol
Catalog No.:BCN7802
CAS No.:333361-85-6
- NBD-556
Catalog No.:BCC1790
CAS No.:333353-44-9
- NBD-557
Catalog No.:BCC1791
CAS No.:333352-59-3
- Etoposide
Catalog No.:BCC1151
CAS No.:33419-42-0
- Quercetin 3,4'-dimethyl ether
Catalog No.:BCN5257
CAS No.:33429-83-3
- 2-Pyridylethylamine dihydrochloride
Catalog No.:BCC7379
CAS No.:3343-39-3
- 3,3'-Bilawsone
Catalog No.:BCN7912
CAS No.:33440-64-1
- Alnustone
Catalog No.:BCN2761
CAS No.:33457-62-4
- Evonine
Catalog No.:BCN3087
CAS No.:33458-82-1
- AH 6809
Catalog No.:BCC1332
CAS No.:33458-93-4
- 3-Aminopiperidine dihydrochloride
Catalog No.:BCC8619
CAS No.:334618-23-4
- Tracheloside
Catalog No.:BCN2738
CAS No.:33464-71-0
- Laburnine
Catalog No.:BCN1992
CAS No.:3348-73-0
- Gisadenafil besylate
Catalog No.:BCC7871
CAS No.:334827-98-4
- HexylHIBO
Catalog No.:BCC7166
CAS No.:334887-43-3
Biological studies on indole derivatives. I. Synthesis and pharmacological studies of 2-oxo-3-indolyl derivatives.[Pubmed:3604703]
Acta Physiol Pharmacol Latinoam. 1986;36(4):391-5.
Two compounds, 3-hydroxy-3-acetonyl-oxindole (I) and compound 3-acetonylidene-oxindole (II) were synthesized by a convenient method, and their pharmacological/toxicological effects were studied in rabbit. These compounds produced transitory effects on serum enzymes. The cholesterol mobilizing effect is more prominent with compound (I).
Synthesis of potential anticonvulsants: condensation of isatins with acetone and related ketones.[Pubmed:6158569]
J Pharm Sci. 1980 Oct;69(10):1235-7.
Substituted isatins were condensed with acetone and other ketones to give analogs of 3-Hydroxy-3-acetonyloxindole. Some of these alcohols were dehydrated. Several compounds with anticonvulsant activity were obtained.


