TrachelosideCAS# 33464-71-0 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 33464-71-0 | SDF | Download SDF |
| PubChem ID | 169511 | Appearance | White powder |
| Formula | C27H34O12 | M.Wt | 550.6 |
| Type of Compound | Lignans | Storage | Desiccate at -20°C |
| Synonyms | 8'-Hydroxyarctigenin 4'-glucoside; 2-Hydroxyarctiin | ||
| Solubility | Soluble in methan | ||
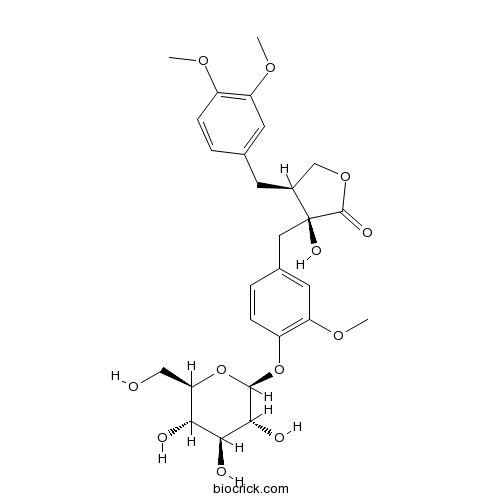
| Chemical Name | (3S,4S)-4-[(3,4-dimethoxyphenyl)methyl]-3-hydroxy-3-[[3-methoxy-4-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxyphenyl]methyl]oxolan-2-one | ||
| SMILES | COC1=C(C=C(C=C1)CC2COC(=O)C2(CC3=CC(=C(C=C3)OC4C(C(C(C(O4)CO)O)O)O)OC)O)OC | ||
| Standard InChIKey | LWYAMIUSVGPFKS-CGLYQLBNSA-N | ||
| Standard InChI | InChI=1S/C27H34O12/c1-34-17-6-4-14(9-19(17)35-2)8-16-13-37-26(32)27(16,33)11-15-5-7-18(20(10-15)36-3)38-25-24(31)23(30)22(29)21(12-28)39-25/h4-7,9-10,16,21-25,28-31,33H,8,11-13H2,1-3H3/t16-,21+,22+,23-,24+,25+,27-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Tracheloside significantly decreases the activity of alkaline phosphatase (AP), an estrogen-inducible marker enzyme, with an IC(50) value of 0.31 microg/ml, a level of inhibition comparable to that of tamoxifen (IC(50) = 0.43 microg/ml). |
| Targets | Estrogen receptor | Progestogen receptor |
| In vitro | An anti-estrogenic lignan glycoside, tracheloside, from seeds of Carthamus tinctorius.[Pubmed: 17090940]Biosci Biotechnol Biochem. 2006 Nov;70(11):2783-5.The lignan glycoside, Tracheloside, was isolated from seeds of Carthamus tinctorius (Compositae) as an anti-estrogenic principle against cultured Ishikawa cells by employing a bioassay-linked HPLC-ELSD method. |
| In vivo | Lack of significant inhibitory effects of a plant lignan tracheloside on 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP)-induced mammary carcinogenesis in female Sprague-Dawley rats.[Pubmed: 14568166]Cancer Lett. 2003 Oct 28;200(2):133-9.Tracheloside, one of the plant lignans which can be extracted from the debris after safflower oil is produced from the seeds of Carthamus tinctorious, is an analogue of another plant lignan, arctiin, the side-chain C-2 of the five-membered ring being changed from a hydrogen to a hydroxyl group. We have already demonstrated that arctiin has chemopreventive effect on mammary carcinogenesis. Therefore, chemopreventive effects of Tracheloside on the initiation or post-initiation period of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP)-induced mammary carcinogenesis in female rats were examined. |

Tracheloside Dilution Calculator

Tracheloside Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.8162 mL | 9.081 mL | 18.162 mL | 36.324 mL | 45.405 mL |
| 5 mM | 0.3632 mL | 1.8162 mL | 3.6324 mL | 7.2648 mL | 9.081 mL |
| 10 mM | 0.1816 mL | 0.9081 mL | 1.8162 mL | 3.6324 mL | 4.5405 mL |
| 50 mM | 0.0363 mL | 0.1816 mL | 0.3632 mL | 0.7265 mL | 0.9081 mL |
| 100 mM | 0.0182 mL | 0.0908 mL | 0.1816 mL | 0.3632 mL | 0.4541 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 3-Aminopiperidine dihydrochloride
Catalog No.:BCC8619
CAS No.:334618-23-4
- AH 6809
Catalog No.:BCC1332
CAS No.:33458-93-4
- Evonine
Catalog No.:BCN3087
CAS No.:33458-82-1
- Alnustone
Catalog No.:BCN2761
CAS No.:33457-62-4
- 3,3'-Bilawsone
Catalog No.:BCN7912
CAS No.:33440-64-1
- 2-Pyridylethylamine dihydrochloride
Catalog No.:BCC7379
CAS No.:3343-39-3
- Quercetin 3,4'-dimethyl ether
Catalog No.:BCN5257
CAS No.:33429-83-3
- Etoposide
Catalog No.:BCC1151
CAS No.:33419-42-0
- 3-Hydroxy-3-acetonyloxindole
Catalog No.:BCN4069
CAS No.:33417-17-3
- Spermidine trihydrochloride
Catalog No.:BCC6865
CAS No.:334-50-9
- (E)-2-Decenoic acid
Catalog No.:BCC1292
CAS No.:334-49-6
- LUF 5834
Catalog No.:BCC6237
CAS No.:333962-91-7
- Laburnine
Catalog No.:BCN1992
CAS No.:3348-73-0
- Gisadenafil besylate
Catalog No.:BCC7871
CAS No.:334827-98-4
- HexylHIBO
Catalog No.:BCC7166
CAS No.:334887-43-3
- (S)-HexylHIBO
Catalog No.:BCC7167
CAS No.:334887-48-8
- MEK inhibitor
Catalog No.:BCC1738
CAS No.:334951-92-7
- Substance P
Catalog No.:BCC6957
CAS No.:33507-63-0
- Fucoxanthin
Catalog No.:BCN2948
CAS No.:3351-86-8
- Luteinizing Hormone Releasing Hormone (LHRH)
Catalog No.:BCC1049
CAS No.:33515-09-2
- 6'-Iodoresiniferatoxin
Catalog No.:BCC7114
CAS No.:335151-55-8
- Bax channel blocker
Catalog No.:BCC2392
CAS No.:335165-68-9
- iMAC2
Catalog No.:BCC2396
CAS No.:335166-36-4
- Raddeanoside 20
Catalog No.:BCN2796
CAS No.:335354-79-5
Lack of significant inhibitory effects of a plant lignan tracheloside on 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP)-induced mammary carcinogenesis in female Sprague-Dawley rats.[Pubmed:14568166]
Cancer Lett. 2003 Oct 28;200(2):133-9.
Tracheloside, one of the plant lignans which can be extracted from the debris after safflower oil is produced from the seeds of Carthamus tinctorious, is an analogue of another plant lignan, arctiin, the side-chain C-2 of the five-membered ring being changed from a hydrogen to a hydroxyl group. We have already demonstrated that arctiin has chemopreventive effect on mammary carcinogenesis. Therefore, chemopreventive effects of Tracheloside on the initiation or post-initiation period of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP)-induced mammary carcinogenesis in female rats were examined. For initiation, female Sprague-Dawley (SD) rats at the 6 weeks of age were given intragastric administrations of 100 mg/kg body weight of PhIP once a week for 8 weeks. The animals were treated with 0.2 or 0.02% Tracheloside during or after this carcinogen exposure. Control rats were fed basal diet with PhIP initiation or 0.2% Tracheloside or basal diet alone without initiation throughout the experimental period. All surviving animals were necropsied at the week 52 of administration. There were no clear treatment-related changes with statistical significance in all parameters for mammary carcinomas measured in this experiment. These results indicate that Tracheloside may not exert significant effects on PhIP-induced mammary carcinogenesis at least under the present experiment condition.
An anti-estrogenic lignan glycoside, tracheloside, from seeds of Carthamus tinctorius.[Pubmed:17090940]
Biosci Biotechnol Biochem. 2006 Nov;70(11):2783-5.
The lignan glycoside, Tracheloside, was isolated from seeds of Carthamus tinctorius (Compositae) as an anti-estrogenic principle against cultured Ishikawa cells by employing a bioassay-linked HPLC-ELSD method. Tracheloside significantly decreased the activity of alkaline phosphatase (AP), an estrogen-inducible marker enzyme, with an IC(50) value of 0.31 microg/ml, a level of inhibition comparable to that of tamoxifen (IC(50) = 0.43 microg/ml).


