NauclefineCAS# 57103-51-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 57103-51-2 | SDF | Download SDF |
| PubChem ID | 320217 | Appearance | Yellow powder |
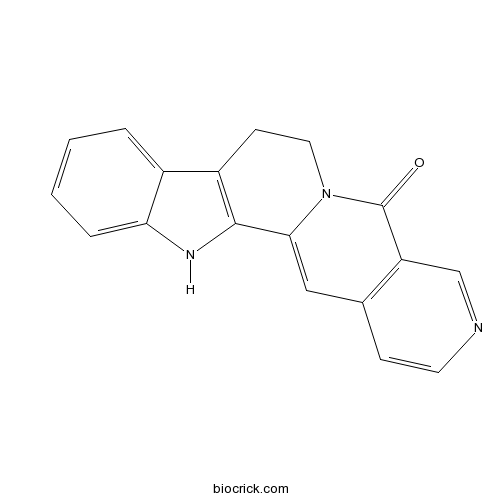
| Formula | C18H13N3O | M.Wt | 287.3 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 3,13,17-triazapentacyclo[11.8.0.02,10.04,9.015,20]henicosa-1(21),2(10),4,6,8,15(20),16,18-octaen-14-one | ||
| SMILES | C1CN2C(=CC3=C(C2=O)C=NC=C3)C4=C1C5=CC=CC=C5N4 | ||
| Standard InChIKey | BGFQUYBVDVRJSP-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C18H13N3O/c22-18-14-10-19-7-5-11(14)9-16-17-13(6-8-21(16)18)12-3-1-2-4-15(12)20-17/h1-5,7,9-10,20H,6,8H2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Nauclefine Dilution Calculator

Nauclefine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.4807 mL | 17.4034 mL | 34.8068 mL | 69.6136 mL | 87.0171 mL |
| 5 mM | 0.6961 mL | 3.4807 mL | 6.9614 mL | 13.9227 mL | 17.4034 mL |
| 10 mM | 0.3481 mL | 1.7403 mL | 3.4807 mL | 6.9614 mL | 8.7017 mL |
| 50 mM | 0.0696 mL | 0.3481 mL | 0.6961 mL | 1.3923 mL | 1.7403 mL |
| 100 mM | 0.0348 mL | 0.174 mL | 0.3481 mL | 0.6961 mL | 0.8702 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- (6E,12E)-Tetradeca-6,12-diene-8,10-diyne-1,3-diol diacetate
Catalog No.:BCN9472
CAS No.:89913-46-2
- (-)-Cedrusin
Catalog No.:BCN9471
CAS No.:404335-99-5
- Trigonothyrin D
Catalog No.:BCN9470
CAS No.:1254956-09-6
- Trigonosin D
Catalog No.:BCN9469
CAS No.:1262842-68-1
- Flavanthrinin
Catalog No.:BCN9468
CAS No.:130827-45-1
- Oxoflaccidin
Catalog No.:BCN9467
CAS No.:121817-24-1
- Lariciresinol p-coumarate
Catalog No.:BCN9466
CAS No.:864452-88-0
- Macedonic acid
Catalog No.:BCN9465
CAS No.:39022-00-9
- Shanciol H
Catalog No.:BCN9464
CAS No.:1114905-55-3
- 2',6'-Dimethoxypaulownin
Catalog No.:BCN9463
CAS No.:115196-22-0
- 4α,8β-Dihydroxy-3α-(2-hydroxy-3-acetoxy-2-methylbutyryloxy)eudesm-7(11)-en-12,8α-olide
Catalog No.:BCN9462
CAS No.:1442989-33-4
- Corialin B
Catalog No.:BCN9461
CAS No.:1325717-47-2
- Trigochinin B
Catalog No.:BCN9474
CAS No.:1210299-32-3
- Imbricatin
Catalog No.:BCN9475
CAS No.:84504-71-2
- Kaempferol 3-O-(4''-O-trans-p-coumaroyl)rhamnopyranoside
Catalog No.:BCN9476
CAS No.:623927-14-0
- Helichrysoside
Catalog No.:BCN9477
CAS No.:56343-26-1
- Pipoxide chlorohydrin
Catalog No.:BCN9478
CAS No.:29228-15-7
- Eudesma-4(15),7(11)-dien-8-one
Catalog No.:BCN9479
CAS No.:54707-47-0
- Jacareubin
Catalog No.:BCN9480
CAS No.:3811-29-8
- 2-Hydroxy-1,8-dimethoxyxanthone
Catalog No.:BCN9481
CAS No.:38974-76-4
- 6-(3-Chloro-2-hydroxy-3-methylbutyl)-5,7-dimethoxycoumarin
Catalog No.:BCN9482
CAS No.:15575-50-5
- Leptostachyol acetate
Catalog No.:BCN9483
CAS No.:35770-58-2
- Rhodomyrtone
Catalog No.:BCN9484
CAS No.:468757-69-9
- Flaccidin
Catalog No.:BCN9485
CAS No.:115531-76-5
An alkaloid initiates phosphodiesterase 3A-schlafen 12 dependent apoptosis without affecting the phosphodiesterase activity.[Pubmed:32591543]
Nat Commun. 2020 Jun 26;11(1):3236.
The promotion of apoptosis in tumor cells is a popular strategy for developing anti-cancer drugs. Here, we demonstrate that the plant indole alkaloid natural product Nauclefine induces apoptosis of diverse cancer cells via a PDE3A-SLFN12 dependent death pathway. Nauclefine binds PDE3A but does not inhibit the PDE3A's phosphodiesterase activity, thus representing a previously unknown type of PDE3A modulator that can initiate apoptosis without affecting PDE3A's canonical function. We demonstrate that PDE3A's H840, Q975, Q1001, and F1004 residues-as well as I105 in SLFN12-are essential for Nauclefine-induced PDE3A-SLFN12 interaction and cell death. Extending these molecular insights, we show in vivo that Nauclefine inhibits tumor xenograft growth, doing so in a PDE3A- and SLFN12-dependent manner. Thus, beyond demonstrating potent cytotoxic effects of an alkaloid natural product, our study illustrates a potentially side-effect-reducing strategy for targeting PDE3A for anti-cancer therapeutics without affecting its phosphodiesterase activity.
Total Synthesis of Camptothecin and Related Natural Products by a Flexible Strategy.[Pubmed:27781350]
Angew Chem Int Ed Engl. 2016 Nov 14;55(47):14778-14783.
A flexible strategy for constructing natural products containing indolizinone or quinolizinone scaffolds and their analogues was developed, which was based on a cascade exo hydroamination followed by spontaneous lactamization. This method was applied in the total synthesis of camptothecin in nine steps in a new ring-forming approach. It was also used to efficiently prepare five biogenetically or structurally related natural alkaloids, including 22-hydroxyacuminatine, oxypalmatine, norketoyobyrine, naucleficine, and Nauclefine, as well as 35 natural-product-like molecules. We believe that this method and the small-molecule library prepared with it can open new avenues for studying the bioactivity of camptothecin and Nauclea natural products.
Natural indole butyrylcholinesterase inhibitors from Nauclea officinalis.[Pubmed:25636869]
Phytomedicine. 2015 Jan 15;22(1):45-8.
Nine monoterpenoid indole alkaloids; naucletine (1), angustidine (2), Nauclefine (3), angustine (4), naucline (5), angustoline (6), harmane (7), 3,14-dihydroangustoline (8), strictosamide (9) and one quinoline alkaloid glycoside; pumiloside (10) from Nauclea officinalis were tested for cholinesterase inhibitory activity. All the alkaloids except for pumiloside (10) showed strong to weak BChE inhibitory effect with IC50 values ranging between 1.02-168.55 muM. Angustidine (2), Nauclefine (3), angustine (4), angustoline (6) and harmane (7) showed higher BChE inhibiting potency compared to galanthamine. Angustidine (2) was the most potent inhibitor towards both AChE and BChE. Molecular docking (MD) studies showed that angustidine (2) docked deep into the bottom gorge of hBChE and formed hydrogen bonding with Ser 198 and His 438. Kinetic study of angustidine (2) on BChE suggested a mixed inhibition mode with an inhibition constant (Ki) of 6.12 muM.
Naucline, a new indole alkaloid from the bark of Nauclea officinalis.[Pubmed:22469596]
Molecules. 2012 Apr 2;17(4):4028-36.
A new indole alkaloid, naucline (1) together with four known alkaloids, angustine (2), angustidine (3), Nauclefine (4) and naucletine (5), were isolated from the bark of Nauclea officinalis. The structures of all isolated compounds were elucidated with various spectroscopic methods such as 1D- and 2D- NMR, IR, UV and LCMS-IT-TOF. In addition to that of alkaloid 1, the complete 13C-NMR data of naucletine (5) were also reported. Naucline (1) showed a moderate vasorelaxant activity (90% relaxation at 1 x 10(-5) M) whereas, angustine (2), Nauclefine (4), and naucletine (5) showed potent vasorelaxant activity (more than 90% relaxation at 1 x 10(-5) M) on an isolated rat aorta.
[Synthesis, physicochemical and pharmacological properties of pentacyclic alkaloid-analogues].[Pubmed:22329301]
Acta Pharm Hung. 2011;81(4):139-49.
Quinazolinocarboline rutaecarpine and evodiamine (Evodia rutaecarpa) are main alkaloid components of traditional Chinese folk-remedies. Evodiamine exhibited selective antitumor and antimetastatic effects on several cancer cell lines and became lead structure of anticancer agents. During our synthetic research we achieved to gain alkaloid hybrid derivatives by combining the structural elements of quinazolinocarbolines with analogous alkaloids or drug molecules having similar effects by bioisosteric replacements. 8-norrutaecarpine, a hybrid molecule of rutaecarpine and luotonin A containing the indolo-pyrroloquinazolinone ring system has been synthesized. The hybrids of rutaecarpine and piroxicam bearing the indolo-pyridobenzothiazine and the 12-azaindolo-pyridobenzothiazine structures were prepared on two alternative routes. Two new heterocondensed pentacyclic compounds, 5-sulfarutaecarpine and 5-sulfa-8-norrutaecarpine were reached via bioisosteric replacement on the structure of rutaecarpine and 8-norrutaecarpine. Two new tricyclic ring systems, pyrido-benzothiadiazine and pyrrolo-benzothiadiazine were produced as intermediaries of these pentacyclic molecules. Series of substituted derivatives were prepared for pharmacological studies by modification of the structures with various substituents and solubilizing groups. During our work alternative way for synthesis of Nauclefine (Nauclea latifolia) was laboured, and we published the synthesis of indolylquinazolinone derivative bouchardatine (Bouchardata neurococca) for the first time. Some of the physicochemical attributes of the synthesized intermediaries were defined, such as the pKa constants of 2,3-poly-methylene-benzothiadiazines. Proton/deuteron exchange kinetic constants of active methylene-groups of five tricyclic compounds were measured by 1H NMR technique. Solvent-dependent ratio of the Z/E isomers of phenyhydrazone-derivatives in polar and apolar solvents were determined. In the case of 18 produced compounds our work was completed by in vitro pharmacological studies performed within co-operation with the Institute of Pharmacology. The viability of HeLa cells was inhibited by five of our compounds to similar extent as the effect of evodiamine. Eight of our compounds induced apoptosis on HeLa cells to similar extent as evodiamine.
[Synthesis of carboline alkaloid analogues].[Pubmed:11862665]
Acta Pharm Hung. 2001 Aug;71(2):171-80.
Hybrid compounds were synthesized combining the structural features of two isomer natural indolalkaloids rutaecarpine (1) and Nauclefine (2). These aza-bioisosteric analogues are the first representatives of a new heterocyclic ring system. Two alternative reaction routes were developed for the synthesis of pentacyclic compounds (4, 5) in which the key step is the Fischer indolization of the 6-phenylhydrazono-dipyrido[1,2-a;4,3-d]pirimidine-11-ones. In the case of E-ring substituted derivatives the synthesis was carried out via preparation and chemical transformation of pyrido[1,2-a]pirimidine-4-ones (14, 15) to 2-substituted-3-aza-rutaecarpines (17-20). Finally, the nucleophilic displacement of the chlorine atom of 2-chloro-3-aza-rutaecarpine (18) by dialkylaminoethylamine provided the 2-amino-substituted derivative (20) having improved physico-chemical properties and increased antitumour activity. The new compounds are characterized by UV, IR, 1H, 13C NMR spectroscopy.
New indole alkaloids from Sarcocephalus latifolius.[Pubmed:11547422]
Nat Prod Lett. 2001;15(1):43-8.
Phytochemical investigation of the root extract of Sarcocephalus latifolius has led to the isolation of the new indole alkaloids 21-O-methylstrictosamide aglycone and 21-O-ethylstrictosamide aglycone, together with strictosamide, angustine, Nauclefine, angustidine, angustoline, 19-O-ethylangustoline, naucleidinal, 19-epi-naucleidinal, quinovic acid-3 beta-O-beta-D-fucopyranoside, quinovic acid-3 beta-O-alpha-L-rhamnopyranoside, scopoletin, and beta-sitosterol. Strictosamide displayed moderate antiplasmodial activity against Plasmodium falciparum.


