HelichrysosideCAS# 56343-26-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 56343-26-1 | SDF | Download SDF |
| PubChem ID | 123132005 | Appearance | Yellow powder |
| Formula | C30H26O14 | M.Wt | 610.5 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
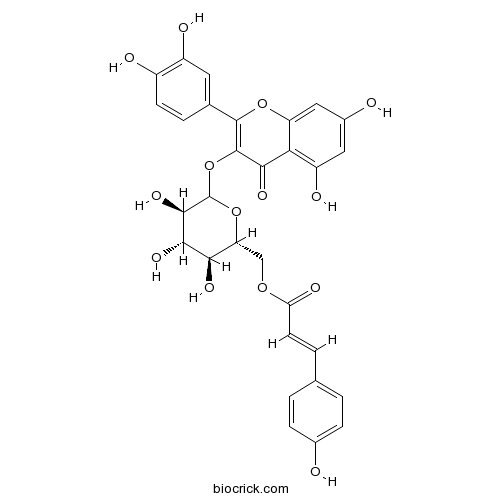
| Chemical Name | [(2R,3S,4S,5R)-6-[2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4-oxochromen-3-yl]oxy-3,4,5-trihydroxyoxan-2-yl]methyl (E)-3-(4-hydroxyphenyl)prop-2-enoate | ||
| SMILES | C1=CC(=CC=C1C=CC(=O)OCC2C(C(C(C(O2)OC3=C(OC4=CC(=CC(=C4C3=O)O)O)C5=CC(=C(C=C5)O)O)O)O)O)O | ||
| Standard InChIKey | NBAZENYUDPJQIH-UPJCVAPKSA-N | ||
| Standard InChI | InChI=1S/C30H26O14/c31-15-5-1-13(2-6-15)3-8-22(36)41-12-21-24(37)26(39)27(40)30(43-21)44-29-25(38)23-19(35)10-16(32)11-20(23)42-28(29)14-4-7-17(33)18(34)9-14/h1-11,21,24,26-27,30-35,37,39-40H,12H2/b8-3+/t21-,24-,26+,27-,30?/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Helichrysoside Dilution Calculator

Helichrysoside Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.638 mL | 8.19 mL | 16.38 mL | 32.76 mL | 40.95 mL |
| 5 mM | 0.3276 mL | 1.638 mL | 3.276 mL | 6.552 mL | 8.19 mL |
| 10 mM | 0.1638 mL | 0.819 mL | 1.638 mL | 3.276 mL | 4.095 mL |
| 50 mM | 0.0328 mL | 0.1638 mL | 0.3276 mL | 0.6552 mL | 0.819 mL |
| 100 mM | 0.0164 mL | 0.0819 mL | 0.1638 mL | 0.3276 mL | 0.4095 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Kaempferol 3-O-(4''-O-trans-p-coumaroyl)rhamnopyranoside
Catalog No.:BCN9476
CAS No.:623927-14-0
- Imbricatin
Catalog No.:BCN9475
CAS No.:84504-71-2
- Trigochinin B
Catalog No.:BCN9474
CAS No.:1210299-32-3
- Nauclefine
Catalog No.:BCN9473
CAS No.:57103-51-2
- (6E,12E)-Tetradeca-6,12-diene-8,10-diyne-1,3-diol diacetate
Catalog No.:BCN9472
CAS No.:89913-46-2
- (-)-Cedrusin
Catalog No.:BCN9471
CAS No.:404335-99-5
- Trigonothyrin D
Catalog No.:BCN9470
CAS No.:1254956-09-6
- Trigonosin D
Catalog No.:BCN9469
CAS No.:1262842-68-1
- Flavanthrinin
Catalog No.:BCN9468
CAS No.:130827-45-1
- Oxoflaccidin
Catalog No.:BCN9467
CAS No.:121817-24-1
- Lariciresinol p-coumarate
Catalog No.:BCN9466
CAS No.:864452-88-0
- Macedonic acid
Catalog No.:BCN9465
CAS No.:39022-00-9
- Pipoxide chlorohydrin
Catalog No.:BCN9478
CAS No.:29228-15-7
- Eudesma-4(15),7(11)-dien-8-one
Catalog No.:BCN9479
CAS No.:54707-47-0
- Jacareubin
Catalog No.:BCN9480
CAS No.:3811-29-8
- 2-Hydroxy-1,8-dimethoxyxanthone
Catalog No.:BCN9481
CAS No.:38974-76-4
- 6-(3-Chloro-2-hydroxy-3-methylbutyl)-5,7-dimethoxycoumarin
Catalog No.:BCN9482
CAS No.:15575-50-5
- Leptostachyol acetate
Catalog No.:BCN9483
CAS No.:35770-58-2
- Rhodomyrtone
Catalog No.:BCN9484
CAS No.:468757-69-9
- Flaccidin
Catalog No.:BCN9485
CAS No.:115531-76-5
- Methoxyimbricatin
Catalog No.:BCN9486
CAS No.:233759-30-3
- 3-O-Debenzoylzeylenone
Catalog No.:BCN9487
CAS No.:1800008-77-8
- 24,24-Dimethyl-5α-lanosta-9(11),25-dien-3β-ol
Catalog No.:BCN9488
CAS No.:33762-29-7
- Vesticarpan
Catalog No.:BCN9489
CAS No.:69853-46-9
Glucose Tolerance-Improving Activity of Helichrysoside in Mice and Its Structural Requirements for Promoting Glucose and Lipid Metabolism.[Pubmed:31847420]
Int J Mol Sci. 2019 Dec 14;20(24). pii: ijms20246322.
An acylated flavonol glycoside, Helichrysoside, at a dose of 10 mg/kg/day per os for 14 days, improved the glucose tolerance in mice without affecting the food intake, visceral fat weight, liver weight, and other plasma parameters. In this study, using hepatoblastoma-derived HepG2 cells, Helichrysoside, trans-tiliroside, and kaempferol 3-O-beta-D-glucopyranoside enhanced glucose consumption from the medium, but their aglycones and p-coumaric acid did not show this activity. In addition, several acylated flavonol glycosides were synthesized to clarify the structural requirements for lipid metabolism using HepG2 cells. The results showed that Helichrysoside and related analogs significantly inhibited triglyceride (TG) accumulation in these cells. The inhibition by Helichrysoside was more potent than that by other acylated flavonol glycosides, related flavonol glycosides, and organic acids. As for the TG metabolism-promoting activity in high glucose-pretreated HepG2 cells, Helichrysoside, related analogs, and their aglycones were found to significantly reduce the TG contents in HepG2 cells. However, the desacyl flavonol glycosides and organic acids derived from the acyl groups did not exhibit an inhibitory impact on the TG contents in HepG2 cells. These results suggest that the existence of the acyl moiety at the 6'' position in the D-glucopyranosyl part is essential for glucose and lipid metabolism-promoting activities.
Anti-oxidative and cholinesterase inhibitory effects of leaf extracts and their isolated compounds from two closely related Croton species.[Pubmed:23377133]
Molecules. 2013 Feb 1;18(2):1916-32.
A comparative evaluation of the antioxidant and acetylcholinesterase inhibitory activity of the leaf extracts of Croton gratissimus and Croton zambesicus (subgratissimus) and compounds isolated from the extracts was carried out to determine their potential and suitability or otherwise as a substitute for each other in the management of oxidative and neurodegenerative conditions. Different antioxidant assays (DPPH, FRAP, beta-carotene-linoleic and the lipid peroxidation models) and the microplate assay for acetylcholinesterase (AChE) inhibition were carried out separately to study the activities of the crude leaf extracts and four solvent fractions from each of the two Croton species. Bioassay guided fractionation was used to target antioxidant constituents of the crude extracts and ethyl acetate fractions of 20% aqueous methanol extract of C. gratissimus on silica gel and Sephadex LH-20 columns resulted in the isolation of kaempferol-3-O-beta-6''(p-coumaroyl) glucopyranoside (tiliroside, 2), apigenin-6-C-glucoside (isovitexin, 3) and kampferol (4). The extract of C. zambesicus yielded quercetin-3-O-beta-6''(p-coumaroyl) glucopyranoside-3'-methyl ether (Helichrysoside- 3'-methyl ether, 1), kaempferol-3-O-beta-6''(p-coumaroyl) glucopyranoside (tiliroside, 2) and apigenin-6-C-glucoside (isovitexin, 3). Three of the isolated compounds and their different combinations were also included in the bioassays. In all the assays performed, the antioxidant capacity and AChE inhibitory effects of C. zambesicus extracts were weaker than those of C. gratissimus. This suggests that C. gratissimus may not be substituted by C. zambesicus, despite the similarity in some of their constituents. Generally, the combinations made from the isolated compounds showed better activities in most of the assays compared to the individual isolated compounds. This suggests mechanisms such as synergism and/or additive effects to be taking place. This study established low, moderate and high antioxidant activities as well as AChE inhibitory effects by the crude extracts, fractions, compounds and compound combinations. This means some of the extracts, isolated compounds and compound combinations could be useful in the management of neurodegenerative conditions and serve as sources of natural neurodegenerative agents.
Isolation and characterisation of novel antioxidant constituents of Croton zambesicus leaf extract.[Pubmed:21762034]
Nat Prod Res. 2011 Aug;25(13):1224-33.
A 1,1-diphenyl-2-picrylhydrazyl (DPPH)-activity-directed fractionation was used to target antioxidant constituents of the ethyl acetate fraction obtained from a 20% aqueous methanol crude extract of Croton zambesicus leaf. Repeated column chromatography of the fraction on silica gel and Sephadex LH-20 led to the isolation of a new natural product, identified as quercetin-3-O-beta-6''(p-coumaroyl) glucopyranoside-3'-methyl ether, Helichrysoside-3'-methyl ether (1), along with kaempferol-3-O-beta-6''(p-coumaroyl) glucopyranoside, tiliroside (2) and apigenin-6-C-glucoside, isovitexin (3) as the antioxidant constituents. The structures of the isolated compounds were elucidated using spectroscopic techniques, namely NMR (1D and 2D) and mass spectrometry. Compounds 1 and 2 are reported from this species for the first time. In the qualitative antioxidant assay, the three isolated compounds instantly bleached the DPPH (0.2% MeOH) purple colour indicating antioxidant activity. In the quantitative antioxidant assay, all the isolated compounds demonstrated weak antioxidant activity compared to quercetin and rutin used as positive control antioxidant agents. The compounds displayed little to no cytotoxicity against Vero cells in an in vitro assay. The presence of these antioxidant compounds in the leaf extract of C. zambesicus could provide a rationale for the ethnomedicinal use of the plant in the management of oxidative-stress-related diseases in folk medicine.
[Chemical constituents of Laggera pterodonta].[Pubmed:20506820]
Zhongguo Zhong Yao Za Zhi. 2010 Mar;35(5):602-6.
OBJECTIVE: To study the chemical constituents of Laggera pterodonta. METHOD: The ethanol extract of L. pterodonta was isolated by column chromatogramphy on silica gel, ODS, and Sephadex LH-20 to afford compounds. The structures of the obtained compounds were identified by chemical reactions and spectroscopic analysis. RESULT: Nineteen compounds were separated and identified to be pterodondiol (1), ilicic acid (2), artemitin (3), chrysosplenetin B (4), 3,5-dihydroxy-3',4',6,7-tetramethoxyflavone (5), chrysosplenol D (6), 5,6,4'-trihydroxy-3,7-dimethoxyflavone (7), quercetin (8), tamarixetin (9), patuletin (10), quercetin-3-O-beta-D-galactopyranoside (11), patuletin-3-O-beta-D-glucopyranoside (12), Helichrysoside (13), 4,5,7-trihydroxy-6-methoxyflavone-3-O-beta-D-rutinoside (14), kaempferol-3-O-beta-D-glucopyranoside (15), stigmasterol (16), stigmasterol 3-O-beta-D-glucopyranoside (17), 2-hydroxy-benzoic acid (18), beta-sitosterol (19). CONCLUSION: Compounds 5, 7, 9-15, and 17-18 were isolated from this plant for the first time. The 13C-NMR data of compound 7 is reported for the first time.
Antihypertensive principles from the leaves of Melastoma candidum.[Pubmed:8255931]
Planta Med. 1993 Oct;59(5):405-7.
Three active principles were isolated from the leaf of Melastoma candidum using the screening of hypotensive effects on spontaneously hypertensive rats (SHR). Intravenous injection of castalagin, procyanidin B-2, or Helichrysoside into SHR lowered the mean blood pressure in a dose-dependent manner, with Helichrysoside being the most potent compound. Plasma noradrenaline (NA) levels, both basal in SHR and elevated in normal rats through cold-stress stimulation, were attenuated by these compounds in a way which was not influenced by adrenalectomy. Decrease of NA release from sympathetic nerves was assumed to be responsible. Moreover, the hypertensive effect of various vasoconstrictors in anesthetized rats was reduced by Helichrysoside. The same results were also observed in castalagin or procyanidin B-2 treated animals. The results indicate that the three principles possess the ability to lower blood pressure through a decrease of sympathetic tone as well as due to direct vasodilatation in SHRs.


