FlavanthrininCAS# 130827-45-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 130827-45-1 | SDF | Download SDF |
| PubChem ID | 14777892 | Appearance | Brown powder |
| Formula | C15H12O3 | M.Wt | 240.25 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
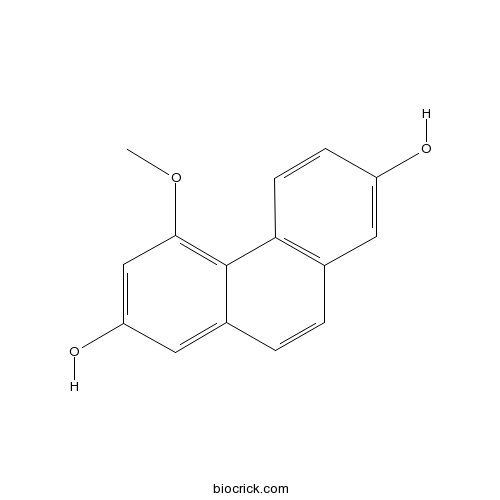
| Chemical Name | 4-methoxyphenanthrene-2,7-diol | ||
| SMILES | COC1=C2C(=CC(=C1)O)C=CC3=C2C=CC(=C3)O | ||
| Standard InChIKey | CJYQJCATAOEZRC-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C15H12O3/c1-18-14-8-12(17)7-10-3-2-9-6-11(16)4-5-13(9)15(10)14/h2-8,16-17H,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Flavanthrinin Dilution Calculator

Flavanthrinin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.1623 mL | 20.8117 mL | 41.6233 mL | 83.2466 mL | 104.0583 mL |
| 5 mM | 0.8325 mL | 4.1623 mL | 8.3247 mL | 16.6493 mL | 20.8117 mL |
| 10 mM | 0.4162 mL | 2.0812 mL | 4.1623 mL | 8.3247 mL | 10.4058 mL |
| 50 mM | 0.0832 mL | 0.4162 mL | 0.8325 mL | 1.6649 mL | 2.0812 mL |
| 100 mM | 0.0416 mL | 0.2081 mL | 0.4162 mL | 0.8325 mL | 1.0406 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Oxoflaccidin
Catalog No.:BCN9467
CAS No.:121817-24-1
- Lariciresinol p-coumarate
Catalog No.:BCN9466
CAS No.:864452-88-0
- Macedonic acid
Catalog No.:BCN9465
CAS No.:39022-00-9
- Shanciol H
Catalog No.:BCN9464
CAS No.:1114905-55-3
- 2',6'-Dimethoxypaulownin
Catalog No.:BCN9463
CAS No.:115196-22-0
- 4α,8β-Dihydroxy-3α-(2-hydroxy-3-acetoxy-2-methylbutyryloxy)eudesm-7(11)-en-12,8α-olide
Catalog No.:BCN9462
CAS No.:1442989-33-4
- Corialin B
Catalog No.:BCN9461
CAS No.:1325717-47-2
- Phrymarolin B
Catalog No.:BCN9460
CAS No.:1363160-29-5
- Toddalolactone 3′-O-methyl ether
Catalog No.:BCN9459
CAS No.:143614-35-1
- Toddalosin ethyl ether
Catalog No.:BCN9458
CAS No.:1538607-31-6
- Schisandrolic acid
Catalog No.:BCN9457
CAS No.:55511-17-6
- Toddalolactone 3′-O-ethyl ether
Catalog No.:BCN9456
CAS No.:1538607-30-5
- Trigonosin D
Catalog No.:BCN9469
CAS No.:1262842-68-1
- Trigonothyrin D
Catalog No.:BCN9470
CAS No.:1254956-09-6
- (-)-Cedrusin
Catalog No.:BCN9471
CAS No.:404335-99-5
- (6E,12E)-Tetradeca-6,12-diene-8,10-diyne-1,3-diol diacetate
Catalog No.:BCN9472
CAS No.:89913-46-2
- Nauclefine
Catalog No.:BCN9473
CAS No.:57103-51-2
- Trigochinin B
Catalog No.:BCN9474
CAS No.:1210299-32-3
- Imbricatin
Catalog No.:BCN9475
CAS No.:84504-71-2
- Kaempferol 3-O-(4''-O-trans-p-coumaroyl)rhamnopyranoside
Catalog No.:BCN9476
CAS No.:623927-14-0
- Helichrysoside
Catalog No.:BCN9477
CAS No.:56343-26-1
- Pipoxide chlorohydrin
Catalog No.:BCN9478
CAS No.:29228-15-7
- Eudesma-4(15),7(11)-dien-8-one
Catalog No.:BCN9479
CAS No.:54707-47-0
- Jacareubin
Catalog No.:BCN9480
CAS No.:3811-29-8
In search of new biological activities of isolates from Odontoglossum Harvengtense 'Tutu'.[Pubmed:23160683]
In Vivo. 2012 Nov-Dec;26(6):993-9.
BACKGROUND: In the current study, we isolated four known compounds, two phenanthrenes, 2,5-dihydroxy-4,9-dimethoxy phenanthrene [1] and 4-methoxyphenanthrene-2,7-diol (Flavanthrinin) [2], one phenanthrenequinone, 5-hydroxy-2,3-dimethoxy-1,4-phenanthrenequinone [3], and one flavone, 3,5,7-trihydroxyflavone (galangin) [4], from the ethyl acetate (EtOAc) extract of Odontoglossum Harvengtense 'Tutu' through bioassay-guided fractionation, and investigated their biological activities. MATERIALS AND METHODS: The isolated compounds were identified with spectroscopic analysis and through comparison to literature values. Cytotoxic activity towards human tumor and normal cells was determined by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) method. Nitric oxide (NO) was determined by the Griess method. Radical scavenging activity was determined by electron spin resonance (ESR) spectroscopy. Osteoclastogenesis was monitored by tartrate-resistant acid phosphatase (TRAP) activity. RESULTS: The compounds had slightly higher cytotoxicity towards human oral squamous cell carcinoma and leukemia cell lines as compared with human normal oral cells, yielding a tumor specificity value of 1.1-2.7. Among these four compounds, 1 most potently inhibited the lipopolysaccharide (LPS)-stimulated NO production and the receptor activator of nuclear factor-kappaB ligand (RANKL)-stimulated osteoclastogenesis by mouse macrophage-like RAW264.7 cells. Micromolar concentrations of 1 scavenged the NO radical produced from 1-hydroxy-2-oxo-3-(N-3-methyl-3-aminopropyl)-3-methyl-1-triazene. CONCLUSION: The present study demonstrated, for the first time, that 1 inhibited both macrophage activation and osteoclast differentiation, suggesting its possible anti-inflammatory action.
Mono-, Bi-, and triphenanthrenes from the tubers of Cremastra appendiculata.[Pubmed:16792409]
J Nat Prod. 2006 Jun;69(6):907-13.
Six newphenanthrene derivatives, including three monophenanthrenes (1-3), two biphenanthrenes (4 and 5), and a triphenanthrene (6), have been isolated from an ethanolic extract of the tubers of Cremastra appendiculata. Using spectroscopic methods, the structures of compounds 1-6 were determined as 1-hydroxy-4,7-dimethoxy-1-(2-oxopropyl)-1H-phenanthren-2-one (1), 1,7-dihydroxy-4-methoxy-1-(2-oxopropyl)-1H-phenanthren-2-one (2), 2-hydroxy-4,7-dimethoxyphenanthrene (3), 2,7,2'-trihydroxy-4,4',7'-trimethoxy-1,1'-biphenanthrene (4), 2,2'-dihydroxy-4,7,4',7'-tetramethoxy-1,1'-biphenanthrene (5), and 2,7,2',7',2' '-pentahydroxy-4,4',4' ',7' '-tetramethoxy-1,8,1',1' '-triphenanthrene (6), respectively. Compounds 1-6 and two known compounds, cirrhopetalanthin (7) and Flavanthrinin (8), obtained previously from this plant, were evaluated against six human cancer cells and a normal cell line.
[Studies on chemical constituents from the corm of Cremastra appendiculata].[Pubmed:16011094]
Zhongguo Zhong Yao Za Zhi. 2005 Apr;30(7):511-3.
OBJECTIVE: To study the chemical constituents of the corm of the planted Cremastra appendiculata. METHOD: The compounds were isolated by column chromatography with silica gel and Sephadex LH-20, and their structures were elucidated by means of spectroscopic methods including 2D NMR techniques. RESULT: Six compounds were isolated, and identified as isohircinol (I), Flavanthrinin (II), p-hydroxyphenylethyl alcohol (III), 3,4-dihydroxyphenylethyl alcohol (IV), daucosterol (V), beta-sitosterol (VI). CONCLUSION: These compounds were not previously isolated from this plant, and isohircinol (I) was obtained from natural source for the first time.


