JacareubinCAS# 3811-29-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 3811-29-8 | SDF | Download SDF |
| PubChem ID | 5281644 | Appearance | Yellow powder |
| Formula | C18H14O6 | M.Wt | 326.3 |
| Type of Compound | Xanthones | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
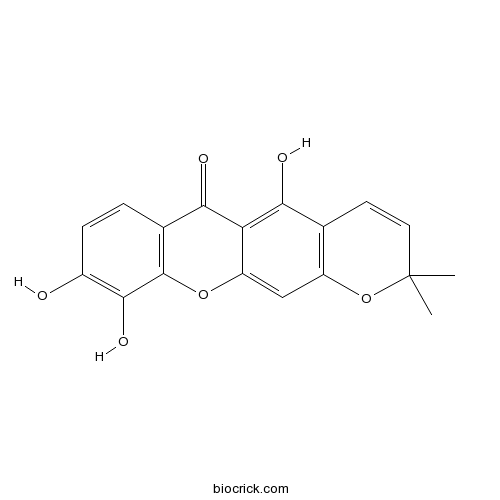
| Chemical Name | 5,9,10-trihydroxy-2,2-dimethylpyrano[3,2-b]xanthen-6-one | ||
| SMILES | CC1(C=CC2=C(O1)C=C3C(=C2O)C(=O)C4=C(O3)C(=C(C=C4)O)O)C | ||
| Standard InChIKey | UCLUVPCGXYTYEK-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C18H14O6/c1-18(2)6-5-8-11(24-18)7-12-13(14(8)20)15(21)9-3-4-10(19)16(22)17(9)23-12/h3-7,19-20,22H,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Jacareubin Dilution Calculator

Jacareubin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.0647 mL | 15.3233 mL | 30.6466 mL | 61.2933 mL | 76.6166 mL |
| 5 mM | 0.6129 mL | 3.0647 mL | 6.1293 mL | 12.2587 mL | 15.3233 mL |
| 10 mM | 0.3065 mL | 1.5323 mL | 3.0647 mL | 6.1293 mL | 7.6617 mL |
| 50 mM | 0.0613 mL | 0.3065 mL | 0.6129 mL | 1.2259 mL | 1.5323 mL |
| 100 mM | 0.0306 mL | 0.1532 mL | 0.3065 mL | 0.6129 mL | 0.7662 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Eudesma-4(15),7(11)-dien-8-one
Catalog No.:BCN9479
CAS No.:54707-47-0
- Pipoxide chlorohydrin
Catalog No.:BCN9478
CAS No.:29228-15-7
- Helichrysoside
Catalog No.:BCN9477
CAS No.:56343-26-1
- Kaempferol 3-O-(4''-O-trans-p-coumaroyl)rhamnopyranoside
Catalog No.:BCN9476
CAS No.:623927-14-0
- Imbricatin
Catalog No.:BCN9475
CAS No.:84504-71-2
- Trigochinin B
Catalog No.:BCN9474
CAS No.:1210299-32-3
- Nauclefine
Catalog No.:BCN9473
CAS No.:57103-51-2
- (6E,12E)-Tetradeca-6,12-diene-8,10-diyne-1,3-diol diacetate
Catalog No.:BCN9472
CAS No.:89913-46-2
- (-)-Cedrusin
Catalog No.:BCN9471
CAS No.:404335-99-5
- Trigonothyrin D
Catalog No.:BCN9470
CAS No.:1254956-09-6
- Trigonosin D
Catalog No.:BCN9469
CAS No.:1262842-68-1
- Flavanthrinin
Catalog No.:BCN9468
CAS No.:130827-45-1
- 2-Hydroxy-1,8-dimethoxyxanthone
Catalog No.:BCN9481
CAS No.:38974-76-4
- 6-(3-Chloro-2-hydroxy-3-methylbutyl)-5,7-dimethoxycoumarin
Catalog No.:BCN9482
CAS No.:15575-50-5
- Leptostachyol acetate
Catalog No.:BCN9483
CAS No.:35770-58-2
- Rhodomyrtone
Catalog No.:BCN9484
CAS No.:468757-69-9
- Flaccidin
Catalog No.:BCN9485
CAS No.:115531-76-5
- Methoxyimbricatin
Catalog No.:BCN9486
CAS No.:233759-30-3
- 3-O-Debenzoylzeylenone
Catalog No.:BCN9487
CAS No.:1800008-77-8
- 24,24-Dimethyl-5α-lanosta-9(11),25-dien-3β-ol
Catalog No.:BCN9488
CAS No.:33762-29-7
- Vesticarpan
Catalog No.:BCN9489
CAS No.:69853-46-9
- (-)-Sesamin 2,2'-diol
Catalog No.:BCN9490
CAS No.:1152441-87-6
- 2,4,3',4',6'-Penta-O-(3-methylbutanoyl)sucrose
Catalog No.:BCN9491
CAS No.:150302-84-4
- Chrysosplenol C
Catalog No.:BCN9492
CAS No.:23370-16-3
Jacareubin inhibits FcepsilonRI-induced extracellular calcium entry and production of reactive oxygen species required for anaphylactic degranulation of mast cells.[Pubmed:29802828]
Biochem Pharmacol. 2018 Aug;154:344-356.
Mast cells (MCs) are important effectors in allergic reactions since they produce a number of pre-formed and de novo synthesized pro-inflammatory compounds in response to the high affinity IgE receptor (FcepsilonRI) crosslinking. IgE/Antigen-dependent degranulation and cytokine synthesis in MCs have been recognized as relevant pharmacological targets for the control of deleterious inflammatory reactions. Despite the relevance of allergic diseases worldwide, efficient pharmacological control of mast cell degranulation has been elusive. In this work, the xanthone Jacareubin was isolated from the heartwood of the tropical tree Callophyllum brasilense, and its tridimensional structure was determined for the first time by X-ray diffraction. Also, its effects on the main activation parameters of bone marrow-derived mast cells (BMMCs) were evaluated. Jacareubin inhibited IgE/Ag-induced degranulation in a dose-response manner with an IC50=46nM. It also blocked extracellular calcium influx triggered by IgE/Ag complexes and by the SERCA ATPase inhibitor thapsigargin (Thap). Inhibition of calcium entry correlated with a blockage on the reactive oxygen species (ROS) accumulation. Antioxidant capacity of Jacareubin was higher than the showed by alpha-tocopherol and caffeic acid, but similar to trolox. Jacareubin shown inhibitory actions on xanthine oxidase, but not on NADPH oxidase (NOX) activities. In vivo, Jacareubin inhibited passive anaphylactic reactions and TPA-induced edema in mice. Our data demonstrate that Jacareubin is a potent natural compound able to inhibit anaphylactic degranualtion in mast cells by blunting FcepsilonRI-induced calcium flux needed for secretion of granule content, and suggest that xanthones could be efficient anti-oxidant, antiallergic, and antiinflammatory molecules.
Cytogenetic effects of Jacareubin from Calophyllum brasiliense on human peripheral blood mononucleated cells in vitro and on mouse polychromatic erythrocytes in vivo.[Pubmed:28943391]
Toxicol Appl Pharmacol. 2017 Nov 15;335:6-15.
Jacareubin is a xanthone isolated from the heartwood of Calophyllum brasiliense with antibacterial and gastroprotective properties and the intention for clinical use as an anti-cancer treatment (due to the similar chemical structure to other anti-neoplastic drugs) requires an investigation of whether this compound can generate adverse effects on non-transformed cells. Jacareubin (0.5-1000muM in DMSO) was more cytotoxic on phytohemagglutinin (PHA)-stimulated normal human peripheral blood mononuclear cells (PBMCs; IC50 at 72h by MTT: 85.9muM) than on G0 phase-PBMCs (IC50 315.6muM) using trypan blue exclusion and formazan metabolism assays. Jacareubin had lower toxicity on PBMCs than Taxol (1muM). Jacareubin presented cytostatic activity because it inhibited PHA-stimulated PBMCs proliferation (from 2.5muM; CFSE dilution and replication index). Jacareubin induced PBMCs arrest in G0/G1 phase of the cell cycle (from 5muM) as evaluated by DNA content. Moreover, Jacareubin generated genotoxicity by breaking DNA strands selectively in PHA-stimulated PBMCs (from 5muM) rather than on resting PBMCs using the single-cell gel electrophoresis assay and increasing the frequency of micronucleated (MN) PBMCs in vitro (from 5muM) and frequency of hypodiploid cells (from 10muM). When 100mg/kg Jacareubin was injected i.p. into mice (a fifth of the LD50; 0.548g/kg. Approximately to 300muM in vitro), we observe no increase in the MN level in bone marrow cells. Jacareubin can be consider for further anti-tumoural activity due to its preferential genotoxic, cytotoxic and cytostatic actions on proliferating cells rather than on resting cells and the lack of in vivo genotoxicity.
Antioxidative compounds from Garcinia buchananii stem bark.[Pubmed:25625705]
J Nat Prod. 2015 Feb 27;78(2):234-40.
An aqueous ethanolic extract of the stem bark of Garcinia buchananii showed strong antioxidative activity using H2O2 scavenging, oxygen radical absorbance capacity (ORAC), and Trolox equivalent antioxidant capacity (TEAC) assays. Activity-guided fractionation afforded three new compounds, isomanniflavanone (1), an ent-eriodictyol-(3alpha-->6)-dihydroquercetin-linked biflavanone, 1,5-dimethoxyaJacareubin (2), and the depsidone garcinisidone-G (3), and six known compounds, (2''R,3''R)-preussianon, euxanthone, 2-isoprenyl-1,3,5,6-tetrahydroxyxanthone, Jacareubin, isogarcinol, and garcinol. All compounds were described for the first time in Garcinia buchananii. The absolute configurations were determined by a combination of NMR, ECD spectroscopy, and polarimetry. These natural products showed high in vitro antioxidative power, especially isomanniflavanone, with an EC50 value of 8.5 muM (H2O2 scavenging), 3.50/4.95 mmol TE/mmol (H/L-TEAC), and 7.54/14.56 mmol TE/mmol (H/L-ORAC).
Antioxidant properties of xanthones from Calophyllum brasiliense: prevention of oxidative damage induced by FeSO(4).[Pubmed:24119308]
BMC Complement Altern Med. 2013 Oct 11;13:262.
BACKGROUND: Reactive oxygen species (ROS) are important mediators in a number of degenerative diseases. Oxidative stress refers to the imbalance between the production of ROS and the ability to scavenge these species through endogenous antioxidant systems. Since antioxidants can inhibit oxidative processes, it becomes relevant to describe natural compounds with antioxidant properties which may be designed as therapies to decrease oxidative damage and stimulate endogenous cytoprotective systems. The present study tested the protective effect of two xanthones isolated from the heartwood of Calophyllum brasilienses against FeSO(4)-induced toxicity. METHODS: Through combinatory chemistry assays, we evaluated the superoxide (O(2).(-)), hydroxyl radical (OH.), hydrogen peroxide (H(2)O(2)) and peroxynitrite (ONO(-)) scavenging capacity of Jacareubin (xanthone III) and 2-(3,3-dimethylallyl)-1,3,5,6-tetrahydroxyxanthone (xanthone V). The effect of these xanthones on murine DNA and bovine serum albumin degradation induced by an OH. generator system was also evaluated. Additionally, we investigated the effect of these xanthones on ROS production, lipid peroxidation and glutathione reductase (GR) activity in FeSO(4)-exposed brain, liver and lung rat homogenates. RESULTS: Xanthone V exhibited a better scavenging capacity for O(2).(-), ONOO(-) and OH. than xanthone III, although both xanthones were unable to trap H(2)O(2). Additionally, xanthones III and V prevented the albumin and DNA degradation induced by the OH. generator system. Lipid peroxidation and ROS production evoked by FeSO(4) were decreased by both xanthones in all tissues tested. Xanthones III and V also prevented the GR activity depletion induced by pro-oxidant activity only in the brain. CONCLUSIONS: Altogether, the collected evidence suggests that xanthones can play a role as potential agents to attenuate the oxidative damage produced by different pro-oxidants.
A new prenylated xanthone from the branches of Calophyllum inophyllum.[Pubmed:21409690]
J Asian Nat Prod Res. 2011 Mar;13(3):265-9.
The investigation of chemical constituents from the branches of Calophyllum inophyllum Linn led to the isolation of a new prenylated xanthone, named caloxanthone Q (1), together with three known compounds, 2-deprenylrheediaxanthone B (2), Jacareubin (3), and 6-deoxyJacareubin (4). Their structures were completely elucidated on the basis of spectroscopic methods (UV, IR, HR-ESI-MS, 1D NMR, and 2D NMR).
[Xanthones from leaves of Calophyllum inophyllum Linn].[Pubmed:19408685]
Yao Xue Xue Bao. 2009 Feb;44(2):154-7.
To study the xanthones from the leaves of Calophyllum inophyllum Linn., several chromatography methods were employed to isolate the constituents. Investigation on the CHCl3 extract led to the isolation of a new xanthone named inophyxanthone A (1) and four known compounds, which were pancixanthone A (2), gerontoxanthone B (3), Jacareubin (4) and pyranoJacareubin (5). Among them, compound 2 was obtained from this plant firstly, and compound 3 was obtained for the first time from this genus. The structure of inophyxanthone A (1) was identified as 1, 3, 5-trihydroxy-2-(1, 1-dimethylallyl)xanthone by spectral analysis.
Antibacterial activity of crude extracts from Mexican medicinal plants and purified coumarins and xanthones.[Pubmed:15707768]
J Ethnopharmacol. 2005 Feb 28;97(2):293-9.
Thirty-two extracts from 22 Mexican medicinal plants of 15 different families were assayed to determine their antibacterial activity against Escherichia coli and Staphylococcus aureus. Seventeen plants showed antibacterial activity, while five plants showed no activity against both bacteria. All of the extracts showed higher activity against Staphylococcus aureus (methicillin-sensitive and methicillin-resistant) than against Escherichia coli, except one. Among the plants examined, Bursera simaruba (L.) Sarg. (Burseraceae), Haematoxylum brasiletto H. Karst. (Fabaceae), Calophyllum brasiliense Cambess. (Clusiaceae), and Mammea americana L. (Clusiaceae) were highly active against Staphylococcus aureus. Coumarins (mammea A/BA and mammea A/AA) and xanthones, namely Jacareubin and 1,3,5,6-tetrahydroxy-2-(3,3-dimethylallyl) xanthone, were isolated as the principle compounds from the last two plants.
Trypanocidal constituents in plants 3. Leaves of Garcinia intermedia and heartwood of Calophyllum brasiliense.[Pubmed:14709920]
Biol Pharm Bull. 2004 Jan;27(1):141-3.
The constituents of the leaves of Garcinia intermedia and heartwood of Calophyllum brasiliense were investigated based on their trypanocidal activity against epimastigotes of Trypanosoma cruzi, the etiologic agent of Chagas' disease. As the active components, the polyisoprenylated benzophenone derivative guttiferone A and the xanthone 8-desoxygartanin were isolated along with the biflavonoids podocarpusflavone A and amentoflavone, and friedelin from the former. Three xanthones, Jacareubin, 6-deoxyJacareubin, and 1,3,5,6-tetrahydroxy-2-(3-methyl-2-butenyl)xanthone from the latter showed activity. The trypanocidal activity of these compounds against trypomastigotes, an infectious form of T. cruzi, was examined as well as gossypol, berberine chloride, and harmine for comparison.


