OuabageninCAS# 508-52-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 508-52-1 | SDF | Download SDF |
| PubChem ID | 12313812 | Appearance | Powder |
| Formula | C23H34O8 | M.Wt | 438.5 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
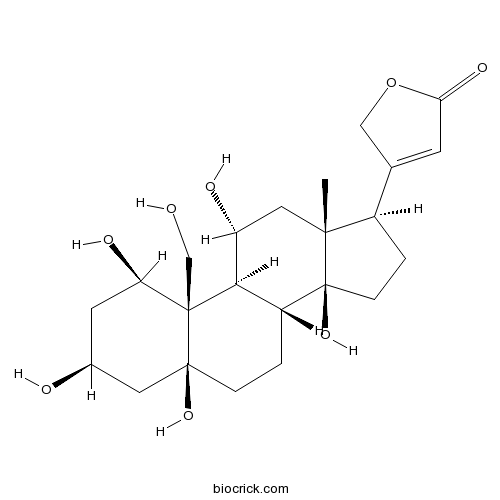
| Chemical Name | 3-[(1R,3S,5S,8R,9S,10R,11R,13R,14S,17R)-1,3,5,11,14-pentahydroxy-10-(hydroxymethyl)-13-methyl-2,3,4,6,7,8,9,11,12,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-17-yl]-2H-furan-5-one | ||
| SMILES | CC12CC(C3C(C1(CCC2C4=CC(=O)OC4)O)CCC5(C3(C(CC(C5)O)O)CO)O)O | ||
| Standard InChIKey | BXSABLKMKAINIU-QOHCMMFCSA-N | ||
| Standard InChI | InChI=1S/C23H34O8/c1-20-9-16(26)19-15(23(20,30)5-3-14(20)12-6-18(28)31-10-12)2-4-21(29)8-13(25)7-17(27)22(19,21)11-24/h6,13-17,19,24-27,29-30H,2-5,7-11H2,1H3/t13-,14+,15+,16+,17+,19+,20+,21-,22+,23-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Ouabagenin Dilution Calculator

Ouabagenin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.2805 mL | 11.4025 mL | 22.805 mL | 45.61 mL | 57.0125 mL |
| 5 mM | 0.4561 mL | 2.2805 mL | 4.561 mL | 9.122 mL | 11.4025 mL |
| 10 mM | 0.2281 mL | 1.1403 mL | 2.2805 mL | 4.561 mL | 5.7013 mL |
| 50 mM | 0.0456 mL | 0.2281 mL | 0.4561 mL | 0.9122 mL | 1.1403 mL |
| 100 mM | 0.0228 mL | 0.114 mL | 0.2281 mL | 0.4561 mL | 0.5701 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- N-acetylanonaine
Catalog No.:BCN2666
CAS No.:5894-74-6
- Glutinone
Catalog No.:BCN5618
CAS No.:508-09-8
- 13(18)-Oleanen-3-ol
Catalog No.:BCN5617
CAS No.:508-04-3
- Oleanolic acid
Catalog No.:BCN5616
CAS No.:508-02-1
- Soyasapogenol A
Catalog No.:BCN1433
CAS No.:508-01-0
- Polyphyllin B
Catalog No.:BCN2833
CAS No.:50773-42-7
- Polyphyllin D
Catalog No.:BCN2401
CAS No.:50773-41-6
- TPCA-1
Catalog No.:BCC2473
CAS No.:507475-17-4
- 3-Cyano-6-isopropylchromone
Catalog No.:BCC8627
CAS No.:50743-32-3
- Vecuronium Bromide
Catalog No.:BCC2498
CAS No.:50700-72-6
- Pennogenin
Catalog No.:BCN2839
CAS No.:507-89-1
- Borneol
Catalog No.:BCN4964
CAS No.:507-70-0
- Rosenonolactone
Catalog No.:BCN5621
CAS No.:508-71-4
- Convallatoxin
Catalog No.:BCC8155
CAS No.:508-75-8
- Hellebrigenol
Catalog No.:BCN8238
CAS No.:508-79-2
- Vasicinol
Catalog No.:BCN5812
CAS No.:5081-51-6
- Astrophylline
Catalog No.:BCN2151
CAS No.:5081-53-8
- Hastatoside
Catalog No.:BCN6898
CAS No.:50816-24-5
- Suchilactone
Catalog No.:BCN6752
CAS No.:50816-74-5
- Ganoderic acid LM2
Catalog No.:BCN2442
CAS No.:508182-41-0
- Vortioxetine
Catalog No.:BCC2046
CAS No.:508233-74-7
- 14,15-Didehydroisoeburnamine
Catalog No.:BCN5619
CAS No.:50838-11-4
- 1,3,5,6-Tetrahydroxyxanthone
Catalog No.:BCN3453
CAS No.:5084-31-1
- Friedelanol
Catalog No.:BCN5620
CAS No.:5085-72-3
Ouabagenin is a naturally occurring LXR ligand without causing hepatic steatosis as a side effect.[Pubmed:29396543]
Sci Rep. 2018 Feb 2;8(1):2305.
Ouabagenin (OBG) is an aglycone of the cardiotonic steroid ouabain and until now was considered a biologically inactive biosynthetic precursor. Herein, we revealed that OBG functions as a novel class of ligand for the liver X receptor (LXR). Luciferase reporter assays and in silico docking studies suggested that OBG has LXR-selective agonistic activity. In addition, OBG repressed the expression of epithelial sodium channel (ENaC), a LXR target gene, without causing hepatic steatosis, a typical side effect of conventional LXR ligands. This remarkable biological activity can be attributed to a unique mode of action; the LXR agonist activity mainly proceeds through the LXRbeta subtype without affecting LXRalpha, unlike conventional LXR ligands. Thus, OBG is a novel class of LXR ligand that does not cause severe side effects, with potential for use as an antihypertensive diuretic or a tool compound for exploring LXR subtype-specific biological functions.
Cardenolide aglycones inhibit tumor necrosis factor alpha-induced expression of intercellular adhesion molecule-1 at the translation step by blocking Na(+)/K(+)-ATPase.[Pubmed:25744456]
Biol Pharm Bull. 2015;38(1):39-47.
Cardiac glycosides, which are inhibitors of Na(+)/K(+)-ATPase, are classified into cardenolides and bufadienolides. We have recently shown that two cardenolide glycosides, ouabain and odoroside A, inhibit Na(+)/K(+)-ATPase, thereby preventing nuclear factor kappaB-inducible protein expression by blocking Na(+)-dependent amino acid transport. In this study, we investigated the mechanism of action of cardenolide aglycones in tumor necrosis factor alpha (TNF-alpha)-induced gene expression. Ouabagenin, digitoxigenin, and digoxigenin were found to inhibit the TNF-alpha-induced cell-surface expression of intercellular adhesion molecule-1 (ICAM-1) in human lung carcinoma A549 cells. Those cardenolide aglycones did not inhibit the TNF-alpha-induced expression of ICAM-1 mRNA, but strongly inhibited the TNF-alpha-induced expression of ICAM-1 as translation product. The inhibition of the TNF-alpha-induced ICAM-1 expression by Ouabagenin, digitoxigenin, and digoxigenin was significantly reversed by the ectopic expression of ouabain-resistant rat Na(+)/K(+)-ATPase alpha1 isoform. Moreover, knockdown of Na(+)/K(+)-ATPase alpha1 isoform augmented the inhibition of the TNF-alpha-induced ICAM-1 expression by Ouabagenin or ouabain. These results clearly indicate that cardenolide aglycones inhibit the TNF-alpha-induced ICAM-1 expression at the translation step by blocking Na(+)/K(+)-ATPase.


