RosenonolactoneCAS# 508-71-4 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 508-71-4 | SDF | Download SDF |
| PubChem ID | 11723309 | Appearance | Powder |
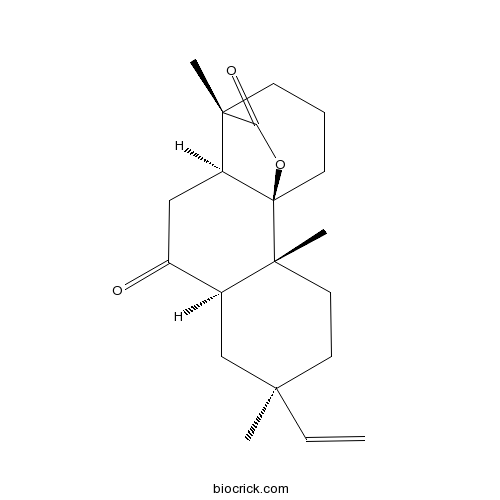
| Formula | C20H28O3 | M.Wt | 316.4 |
| Type of Compound | Diterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| SMILES | CC1(CCC2(C(C1)C(=O)CC3C24CCCC3(C(=O)O4)C)C)C=C | ||
| Standard InChIKey | HWECMADGHQKSLK-BWXAORSUSA-N | ||
| Standard InChI | InChI=1S/C20H28O3/c1-5-17(2)9-10-19(4)13(12-17)14(21)11-15-18(3)7-6-8-20(15,19)23-16(18)22/h5,13,15H,1,6-12H2,2-4H3/t13-,15-,17+,18-,19+,20+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Rosenonolactone shows inhibitory activity against prolyl endopeptidase and thrombin. |

Rosenonolactone Dilution Calculator

Rosenonolactone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.1606 mL | 15.8028 mL | 31.6056 mL | 63.2111 mL | 79.0139 mL |
| 5 mM | 0.6321 mL | 3.1606 mL | 6.3211 mL | 12.6422 mL | 15.8028 mL |
| 10 mM | 0.3161 mL | 1.5803 mL | 3.1606 mL | 6.3211 mL | 7.9014 mL |
| 50 mM | 0.0632 mL | 0.3161 mL | 0.6321 mL | 1.2642 mL | 1.5803 mL |
| 100 mM | 0.0316 mL | 0.158 mL | 0.3161 mL | 0.6321 mL | 0.7901 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Ouabagenin
Catalog No.:BCC8227
CAS No.:508-52-1
- N-acetylanonaine
Catalog No.:BCN2666
CAS No.:5894-74-6
- Glutinone
Catalog No.:BCN5618
CAS No.:508-09-8
- 13(18)-Oleanen-3-ol
Catalog No.:BCN5617
CAS No.:508-04-3
- Oleanolic acid
Catalog No.:BCN5616
CAS No.:508-02-1
- Soyasapogenol A
Catalog No.:BCN1433
CAS No.:508-01-0
- Polyphyllin B
Catalog No.:BCN2833
CAS No.:50773-42-7
- Polyphyllin D
Catalog No.:BCN2401
CAS No.:50773-41-6
- TPCA-1
Catalog No.:BCC2473
CAS No.:507475-17-4
- 3-Cyano-6-isopropylchromone
Catalog No.:BCC8627
CAS No.:50743-32-3
- Vecuronium Bromide
Catalog No.:BCC2498
CAS No.:50700-72-6
- Pennogenin
Catalog No.:BCN2839
CAS No.:507-89-1
- Convallatoxin
Catalog No.:BCC8155
CAS No.:508-75-8
- Hellebrigenol
Catalog No.:BCN8238
CAS No.:508-79-2
- Vasicinol
Catalog No.:BCN5812
CAS No.:5081-51-6
- Astrophylline
Catalog No.:BCN2151
CAS No.:5081-53-8
- Hastatoside
Catalog No.:BCN6898
CAS No.:50816-24-5
- Suchilactone
Catalog No.:BCN6752
CAS No.:50816-74-5
- Ganoderic acid LM2
Catalog No.:BCN2442
CAS No.:508182-41-0
- Vortioxetine
Catalog No.:BCC2046
CAS No.:508233-74-7
- 14,15-Didehydroisoeburnamine
Catalog No.:BCN5619
CAS No.:50838-11-4
- 1,3,5,6-Tetrahydroxyxanthone
Catalog No.:BCN3453
CAS No.:5084-31-1
- Friedelanol
Catalog No.:BCN5620
CAS No.:5085-72-3
- Tetramisole HCl
Catalog No.:BCC4735
CAS No.:5086-74-8
Enzyme inhibitory constituents from Duranta repens.[Pubmed:11964000]
Chem Pharm Bull (Tokyo). 2002 Apr;50(4):515-8.
Isoprenylated flavonoids 5,7-dihydroxy-3'-(2-hydroxy-3-methyl-3-butenyl)-3,6,4'-trimethoxyflavone (1), 3,7-dihydroxy-3'-(2-hydroxy-3-methyl-3-butenyl)-5,6,4'-trimethoxyflavone (2) and an isoprenylated acetophenone derivative (3) have been isolated from Duranta repens along with known compounds, 5-hydroxy-3,6,7,4'-tetramethoxyflavone (4), Rosenonolactone (5), 6,7-dimethoxycoumarin (6), 5alpha,8alpha-epidioxyergosta-6,22-dien-3beta-ol (7) and 5alpha,8alpha-epidioxyergosta-6,9(11),22-trien-3beta-ol (8), isolated for the first time from this species. Their structures and the relative configuration were determined by spectroscopic methods (1H- and 13C-NMR, IR, UV and MS) and two-dimensional (2D)-NMR experiments. The compounds 1-5 showed inhibitory activity against prolyl endopeptidase while 4 and 5 were also active against thrombin.
DNA strand break induction, mutagenicity, and cytotoxicity of the mycotoxins 11-beta-hydroxy-7-deoxy-rosenonolactone, rosenonolactone, and trichothecin.[Pubmed:23606003]
Mycotoxin Res. 1992 Sep;8(2):77-83.
11-beta-hydroxy-7-deoxy-Rosenonolactone (TSS1), a mycotoxin of the rosenane class, was tested on cytotoxicity, induction of DNA single strand breaks and muta-genicity. Its effects were compared to those of Rosenonolactone and trichothecin. TSS1 had stronger antibiotic activity againstEscherichia coli (EC 50: 10mug/mL) than Rosenonolactone (EC 50: >200mug/mL) but weaker activity than trichothecin (EC 50: 3mug/mL). The same order of activity was found for the inhibition of yeast fermentation (EC 50 of TSS1: 45mug/mL; EC 50 of Rosenonolactone: > 120mug/mL; EC 50 of trichothecin: 3.4mug/mL).In the trypan blue exclusion test using V79 Chinese hamster cells, TSS1 proved to be cytotoxic (EC50: 30mug/mL) at even lower doses than trichothecin (EC50: 200mug/mL). Rosenonolactone had no significant toxicity up to the highest soluble concentration (500mug/mL).DNA single strand breaks caused by TSS1 occurred at the same concentrations at which damage of the cell membrane became apparent. For trichothecin single strand breaks were detected only at concentrations at which the membrane was already highly damaged. No single strand breaks were observed in V79 cells after incubation with Rosenonolactone up to the limit of solubility (500mug/mL).In the reversion assay withhis Salmonella typhimurium strains TA 98 and TA 100, no mutagenicity was observed for any of the examined mycotoxins up to 800mug/plate with and without the addition of a rat liver preparation for metabolism of the test compound.


